Abstract
The recently published genome of the unicellular red alga Porphyridium purpureum revealed a gene-rich, intron-poor species, which is surprising for a free-living mesophile. Of the 8,355 predicted protein-coding regions, up to 773 (9.3%) were implicated in horizontal genetic transfer (HGT) events involving other prokaryote and eukaryote lineages. A much smaller number, up to 174 (2.1%) showed unambiguous evidence of vertical inheritance. Together with other red algal genomes, nearly all published in 2013, these data provide an excellent platform for studying diverse aspects of algal biology and evolution. This novel information will help investigators test existing hypotheses about the impact of endosymbiosis and HGT on algal evolution and enable comparative analysis within a more-refined, hypothesis-driven framework that extends beyond HGT. Here we explore the impacts of this infusion of red algal genome data on addressing questions regarding the complex nature of algal evolution and highlight the need for scalable phylogenomic approaches to handle the forthcoming deluge of sequence information.
The recent report of the Porphyridium purpureum genomeCitation1 is the first from a unicellular mesophilic red alga, i.e., a cell that lives in what we consider a “normal” environment in terms of temperature, toxic metal concentrations, humidity, and acidity. The previously determined genomes of unicellular red algae were all from extremophiles that exist in acidic and high temperature habitats around hot springs (i.e., Cyanidioschyzon merolae,Citation2 Galdieria sulphuraria,Citation3 and Galdieria phlegreaCitation4), and thus are highly reduced and specialized to their hostile environment. In addition, genomes from two multicellular red algal species, Chondrus crispusCitation5 and Pyropia yezoensis,Citation6 have recently become available. These genome data, together with other transcriptome data,Citation7,Citation8 provide a significant boost to the currently available genetic repertoire from red algae. The rapid growth in these data therefore inspires a fresh look at the evolutionary history of genes in the red algal lineage.
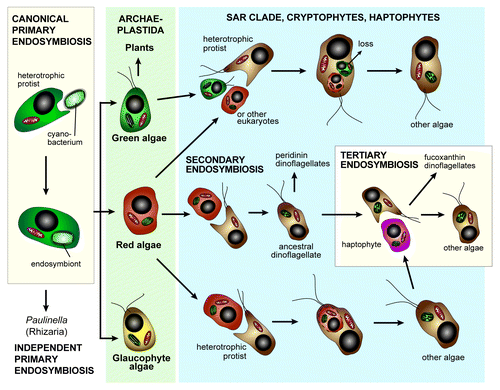
Red algae (Rhodophyta), together with glaucophyte and green algae (the latter gave rise to plants), form the foundational lineage of all photosynthetic eukaryotes, the Archaeplastida or PlantaeCitation9-Citation11 (). Some red algal lineages are economically important due to their yield of hydrocolloid compounds, e.g., agar, agarose, and carrageenan as thickening and emulsifying agents in a wide range of industries.Citation12 As an anciently diverged group of photosynthetic eukaryotes, red algae are also key mediators of horizontal genetic transfer (HGT), directly or indirectly via endosymbiosis.Citation13 This is because an ancestral red alga-like cell is believed to have been implicated in the landmark secondary endosymbiosis event that gave rise to the plastids in a diverse collection of photosynthetic eukaryotesCitation14-Citation16 (). Following endosymbiosis hundreds of red algal genes migrated to the nucleus of the host through a specific form of HGT referred to as endosymbiotic gene transfer (EGT).Citation17 This process spread red algal genes throughout many photosynthetic branches in the eukaryotic tree of life.Citation16,Citation18 Some of these branches, such as the SAR clade (stramenopiles, alveolates, and rhizariansCitation19), cryptophytes, and haptophytes also underwent additional (some putatively cryptic; see below) endosymbioses and other forms of HGT (i.e., genomic “footprints” exist of these events) that have significantly modified their gene inventory.Citation20-Citation24
Figure 1. Plastid primary, secondary, and tertiary (only in dinoflagellates) endosymbiosis and horizontal genetic transfer in the evolution of photosynthetic eukaryotes. The rhizarian Paulinella gained its cyanobacterium-derived plastid from an independent, more recent primary endosymbiosis than the one that gave rise to the canonical Archaeplastida plastid. The origin of the red plastid in the SAR clade and in cryptophytes and haptophytes led to endosymbiotic genetic transfer (a specific form of HGT) of red algal genes to the nuclear genome of these taxa, whereas a cryptic green algal endosymbiosis may explain the presence of hundreds of green algal derived genes in these same lineages. The phylogenetic relationship of the SAR clade to cryptophytes and haptophytes remains unresolved.
Studies of red algal genomes and transcriptomes have yielded novel insights into red algal biology and physiology, including the role of sodium-coupled membrane transport systems,Citation25 the cellular localization of fatty acid biosynthesis, and elucidation of carbohydrate biosynthesis and metabolism.Citation7 A surprising feature of the genomes of mesophilic red algae, perhaps most unexpectedly in multicellular species, is high gene density with few introns, e.g., average of 0.3 and 0.7 introns per gene respectively in Chondrus crispusCitation5 and Pyropia yezoensis.Citation6 The recent analysis of P. purpureumCitation1 reveals a small (19.7 Mbp) genome containing only about 8,355 genes with 235 spliceosomal introns.
Red Algal Evolution Beyond Horizontal Genetic Transfer
From the perspective of evolutionary biology, analysis of 8,355 predicted genesCitation1 in P. purpureum reveals two important aspects. First, 103 (1.2% of 8,355) genes show a strong signal of vertical inheritance, based on a stringent bootstrap support at ≥ 90% for a monophyletic clade in a tree, with an evolutionary association with genes from glaucophyte and green algae (and plants); these trees provide evidence of Archaeplastida monophyly. This number becomes 174 (2.1%) at the less-stringent bootstrap support of ≥ 70%. Second, at least 453 (5.4% of 8,355) genes (bootstrap ≥ 90%), and up to 773 (9.3%; bootstrap ≥ 70%), show a strong signal of reticulate evolution with a clear association of red algal genes with other distantly related photosynthetic eukaryotes and prokaryotes. These latter findings are largely consistent with earlier studies.Citation3,Citation26 The prokaryote footprints in red algal genomes are most often represented by Cyanobacteria (i.e., primarily EGT from the plastid endosymbiont), Proteobacteria, and Chlamydiae.Citation27,Citation28 Because ancestral red algal lineages play a central role in the origin of plastids across many important microbial eukaryotes (e.g., SAR clade; ), the extent of HGT has largely been associated with endosymbiosis (EGT). Interestingly, the number of P. purpureum genes that are implicated overall by HGT is approximately 5-fold greater than those providing clear evidence of Archaeplastida monophyly (i.e., vertical inheritance).
Most studies of algal evolution have relied on standard phylogenomic approaches to infer evolutionary relationships among a set of homologous genes or proteins, either using a gene-by-gene or a concatenated multi-gene approach. A highly provocative hypothesis in eukaryote evolution that came as a result of the gene-by-gene approach concerns the origin of red and/or green algal derived genes in stramenopiles (e.g., the ubiquitous diatoms) and other chlorophyll c-containing algae such as in the SAR clade. The finding of hundreds of green algal derived genes in these taxa, most of which contain a red algal derived plastid, was suggested to have arisen from a cryptic green algal endosymbiosis that predated the red algal capture.Citation29,Citation30 Others have attributed the significant numbers of “green genes” in SAR and other chlorophyll c-containing algae to inadequate sampling of red algal genomes in phylogenomic analyses.Citation31,Citation32 Under this rationale, the inclusion of a larger red algal gene repertoire would clarify the vertical vs. horizontal signals of evolution in microbial eukaryotes. In these instances, a vertical signal would be observed in cases where green algal genes are also present in red algae, whereby a horizontal signal would manifest as green algal genes that are not present in red algae, or if present, do not interrupt the robust monophyly of green and chlorophyll c-containing algae. The net result of the addition of red algal data to the analysis would therefore be to significantly reduce the green footprint. However, simply having more red algal genes in the analysis did not resolve the debate in favor of rampant HGT being the sole reason for green gene presence. Recent studies that include the novel red algal genome data in the analysis do not show a significant reduction in the green gene footprint.Citation30,Citation33,Citation34 Furthermore, analysis of diatom genomes reveals green algal derived genes to encode functions relevant to plastid metabolism such as multiple components of the carotenoid biosynthesis pathway,Citation35 photoprotection under fluctuating light conditions,Citation36 membrane transport,Citation33 and fatty acid biosynthesis.Citation37 These might be the sorts of highly beneficial functions that would be maintained as remnants of a previous plastid of green algal provenance. In summary, HGT between ancestral green algal and chlorophyll c-containing lineages remains the most parsimonious (thus the most plausible) explanation for the presence of green genes in the SAR (and potentially other) clades. The question of whether some or most of these genes arose via a cryptic green algal endosymbiosis, or all owe their origin to unprecedented levels of HGTs, remains to be resolved. It may be the case that both endosymbiosis and other forms of HGT explain the complex distribution of green genes found thus far in these anciently diverged lineages.Citation29,Citation30
Genome evolution, of course, extends beyond foreign gene acquisition, as well as beyond gene boundaries. Genome innovation can be driven by mutation, genetic rearrangement and adaptive (i.e. neutral, positive, or negative) selection.Citation38 These events could leave a more-prominent footprint on algal genomes compared with that of horizontal and/or endosymbiotic genetic transfer. In fact, the former is likely to obscure signals of horizontal inheritance through genome amelioration.Citation39 Until very recently, we had limited genome data to explore such issues.
The availability in 2013 of genomes from mesophilic red algal species is a significant development for the algal research community, because these genomes allow for comparative analysis without the restriction of the sole, highly reduced genome of the hyperthermophilic red alga C. merolaeCitation2—a situation “plaguing” the community for almost a decade. Perhaps more importantly, these genome data provide timely complements and references for validation for other omic data such as transcriptomes, proteomes, epigenomes, and metabolomes. Cross-data set examination allows for an integrated, systems biology approach to study diverse aspects of algal biology and evolution. Various aspects of genome innovation in red algae and their relevance to other photosynthetic species can be investigated via hypothesis-driven experiments, including but not limited to genome expansion, sub-, neo-, or dys-functionalization of genetic elements, and the contribution of repetitive and epigenetic elements to adaptation of algae to the environment. Specifically relevant to red algae, key biological questions surround the origin and development of sexual reproduction, cellular complexity and ploidies across diverse lineages.
Future Perspectives
With the advancement of next-generation sequencing technologies, there is an on-going deluge of sequence data. BGI for instance has launched its Million Genomes Projects (http://www.genomics.cn/en/) to generate reference genomes for thousands of human, animal, and plant species. To generate a finished genome is costly in terms of funding, time and expert resources. Large and mega-genome projects are geared toward sequencing to high-depth, rather than finished genomes; e.g., the Genome Encyclopedia of Bacteria and Archaea Project at the Joint Genome Institute (http://www.jgi.doe.gov/programs/GEBA/), and the 1001 Genome Projects (http://www.1001genomes.org). Similarly, in the case of red algae for which most genomes are sequenced and assembled de novo as drafts, the genome data remain fragmented. No finished red algal genomes (except for C. merolae) are available to date, and given the current challenging funding climate worldwide, a costly investment simply to generate finished red algal genomes is unlikely to be attractive to funding agencies. Researchers are also not likely to endorse this approach given the increasing pressure to quickly produce results using currently available data.
The generation of genome data that adequately address the taxonomic breadth of photosynthetic eukaryotes remains a challenge. Such data are ideal for a comprehensive investigation of the impact of HGT on the evolution of these species, and of photosynthesis in general. Whereas it might seem straightforward to sequence the genome of every living species under the sun, technical challenges remain for many idiosyncratic algal genomes, such as those from dinoflagellates, which are of immense size (some estimated > 200 Gbp)Citation40,Citation41 and exhibit anomalous features including non-canonical nucleotides and unusual intron-exon splice signals.Citation42 The seemingly trivial issue of nucleic acid extraction remains challenging for recalcitrant algal species.Citation43,Citation44 In addition, the issue of sampling biases is inevitable, and unlikely to be resolved in the near future. The appealing idea that simply having more data will lead to the resolution of phylogenetic relationships in the tree of life remains to be validated in the real world.Citation45,Citation46
Unfinished draft genomes comprised of fragmented assemblies are not uncommon. Currently and soon-to-be available genome data are expected to be far more abundant but noisier than those of model genomes. In light of this situation, limitations of using multiple sequence alignment in the standard phylogenomic approach, and its scalability to handle large amounts of sequence data have been highlighted recently.Citation47 An alternative to multiple sequence alignment is to use subsequences at predefined length to reconstruct sequence distances, which can then directly be used to infer phylogenetic relationships,Citation48,Citation49 or identify HGT events.Citation50 This alternative approach is potentially scalable, because it does not require fulfilling the hypothesis of positional homology that is central to techniques such as maximum parsimony, maximum likelihood, or Bayesian inference, most of which are computationally intensive and time-consuming. As the approach bypasses the implicit assumption of full-length contiguity of homologous sequences, it is less sensitive to genetic rearrangement than multiple sequence alignment, reducing loss of phylogenetic information. Although the robustness and scalability of these approaches remain to be systematically investigated, a highly scalable strategy in phylogenomics remains attractive as more sequence data arrive on the scene.
No matter which approach one decides on using, having genome data of sufficient quality is key. Whereas it remains unclear if having more genome data from red algae will help resolve most or all controversies in eukaryote evolution, the novel data clearly show great potential in accelerating discoveries in algal biology and evolution that extend beyond HGT.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
CXC is supported by a ResTeach Fellowship from The University of Queensland. The red algal genome research was supported by a grant from the National Science Foundation awarded to DB (0936884).
Published online: 01/02/2014
Citation: Chan CX, Bhattacharya D. Analysis of horizontal genetic transfer in red algae in the post-genomics age. Mobile Genetic Elements 2014; 3:e27669; 10.4161/mge.27669
References
- Bhattacharya D, Price DC, Chan CX, Qiu H, Rose N, Ball S, Weber AP, Arias MC, Henrissat B, Coutinho PM, et al. Genome of the red alga Porphyridium purpureum.. Nat Commun 2013; 4:1941; http://dx.doi.org/10.1038/ncomms2931; PMID: 23770768
- Matsuzaki M, Misumi O, Shin-I T, Maruyama S, Takahara M, Miyagishima SY, Mori T, Nishida K, Yagisawa F, Nishida K, et al. Genome sequence of the ultrasmall unicellular red alga Cyanidioschyzon merolae 10D. Nature 2004; 428:653 - 7; http://dx.doi.org/10.1038/nature02398; PMID: 15071595
- Schönknecht G, Chen WH, Ternes CM, Barbier GG, Shrestha RP, Stanke M, Bräutigam A, Baker BJ, Banfield JF, Garavito RM, et al. Gene transfer from bacteria and archaea facilitated evolution of an extremophilic eukaryote. Science 2013; 339:1207 - 10; http://dx.doi.org/10.1126/science.1231707; PMID: 23471408
- Qiu H, Price DC, Weber APM, Reeb V, Yang EC, Lee JM, Kim SY, Yoon HS, Bhattacharya D. Adaptation through horizontal gene transfer in the cryptoendolithic red alga Galdieria phlegrea.. Curr Biol 2013; 23:R865 - 6; http://dx.doi.org/10.1016/j.cub.2013.08.046; PMID: 24112977
- Collén J, Porcel B, Carré W, Ball SG, Chaparro C, Tonon T, Barbeyron T, Michel G, Noel B, Valentin K, et al. Genome structure and metabolic features in the red seaweed Chondrus crispus shed light on evolution of the Archaeplastida. Proc Natl Acad Sci U S A 2013; 110:5247 - 52; http://dx.doi.org/10.1073/pnas.1221259110; PMID: 23503846
- Nakamura Y, Sasaki N, Kobayashi M, Ojima N, Yasuike M, Shigenobu Y, Satomi M, Fukuma Y, Shiwaku K, Tsujimoto A, et al. The first symbiont-free genome sequence of marine red alga, Susabi-nori (Pyropia yezoensis). PLoS One 2013; 8:e57122; http://dx.doi.org/10.1371/journal.pone.0057122; PMID: 23536760
- Chan CX, Blouin NA, Zhuang Y, Zäuner S, Prochnik SE, Lindquist E, et al. Porphyra (Bangiophyceae) transcriptomes provide insights into red algal development and metabolism. J Phycol 2012; 48:1328 - 42; http://dx.doi.org/10.1111/j.1529-8817.2012.01229.x
- Xie C, Li B, Xu Y, Ji D, Chen C. Characterization of the global transcriptome for Pyropia haitanensis (Bangiales, Rhodophyta) and development of cSSR markers. BMC Genomics 2013; 14:107; http://dx.doi.org/10.1186/1471-2164-14-107; PMID: 23414227
- Cavalier-Smith T. A revised six-kingdom system of life. Biol Rev Camb Philos Soc 1998; 73:203 - 66; http://dx.doi.org/10.1017/S0006323198005167; PMID: 9809012
- Rodríguez-Ezpeleta N, Brinkmann H, Burey SC, Roure B, Burger G, Löffelhardt W, Bohnert HJ, Philippe H, Lang BF. Monophyly of primary photosynthetic eukaryotes: green plants, red algae, and glaucophytes. Curr Biol 2005; 15:1325 - 30; http://dx.doi.org/10.1016/j.cub.2005.06.040; PMID: 16051178
- Price DC, Chan CX, Yoon HS, Yang EC, Qiu H, Weber AP, Schwacke R, Gross J, Blouin NA, Lane C, et al. Cyanophora paradoxa genome elucidates origin of photosynthesis in algae and plants. Science 2012; 335:843 - 7; http://dx.doi.org/10.1126/science.1213561; PMID: 22344442
- Bixler HJ, Porse H. A decade of change in the seaweed hydrocolloids industry. J Appl Phycol 2011; 23:321 - 35; http://dx.doi.org/10.1007/s10811-010-9529-3
- Margulis L. Origin of Eukaryotic Cells. New Haven: Yale University Press, 1970
- Cavalier-Smith T. The kingdom Chromista: origin and systematics. In: Round FE, Chapman DJ, eds. Progress in Phycological Research. Bristol, UK: Biopress Ltd, 1986
- Cavalier-Smith T. Principles of protein and lipid targeting in secondary symbiogenesis: euglenoid, dinoflagellate, and sporozoan plastid origins and the eukaryote family tree. J Eukaryot Microbiol 1999; 46:347 - 66; http://dx.doi.org/10.1111/j.1550-7408.1999.tb04614.x; PMID: 18092388
- Keeling PJ. Chromalveolates and the evolution of plastids by secondary endosymbiosis. J Eukaryot Microbiol 2009; 56:1 - 8; http://dx.doi.org/10.1111/j.1550-7408.2008.00371.x; PMID: 19335769
- Martin W, Stoebe B, Goremykin V, Hapsmann S, Hasegawa M, Kowallik KV. Gene transfer to the nucleus and the evolution of chloroplasts. Nature 1998; 393:162 - 5; http://dx.doi.org/10.1038/30234; PMID: 11560168
- Janouškovec J, Horák A, Oborník M, Lukes J, Keeling PJ. A common red algal origin of the apicomplexan, dinoflagellate, and heterokont plastids. Proc Natl Acad Sci U S A 2010; 107:10949 - 54; http://dx.doi.org/10.1073/pnas.1003335107; PMID: 20534454
- Burki F, Shalchian-Tabrizi K, Minge M, Skjaeveland A, Nikolaev SI, Jakobsen KS, Pawlowski J. Phylogenomics reshuffles the eukaryotic supergroups. PLoS One 2007; 2:e790; http://dx.doi.org/10.1371/journal.pone.0000790; PMID: 17726520
- Yoon HS, Hackett JD, Van Dolah FM, Nosenko T, Lidie KL, Bhattacharya D. Tertiary endosymbiosis driven genome evolution in dinoflagellate algae. Mol Biol Evol 2005; 22:1299 - 308; http://dx.doi.org/10.1093/molbev/msi118; PMID: 15746017
- Baurain D, Brinkmann H, Petersen J, Rodríguez-Ezpeleta N, Stechmann A, Demoulin V, Roger AJ, Burger G, Lang BF, Philippe H. Phylogenomic evidence for separate acquisition of plastids in cryptophytes, haptophytes, and stramenopiles. Mol Biol Evol 2010; 27:1698 - 709; http://dx.doi.org/10.1093/molbev/msq059; PMID: 20194427
- Chan CX, Bhattacharya D, Reyes-Prieto A. Endosymbiotic and horizontal gene transfer in microbial eukaryotes: Impacts on cell evolution and the tree of life. Mob Genet Elements 2012; 2:101 - 5; http://dx.doi.org/10.4161/mge.20110; PMID: 22934244
- Burki F, Okamoto N, Pombert JF, Keeling PJ. The evolutionary history of haptophytes and cryptophytes: phylogenomic evidence for separate origins. Proc Biol Sci 2012; 279:2246 - 54; http://dx.doi.org/10.1098/rspb.2011.2301; PMID: 22298847
- Moszczyński K, Mackiewicz P, Bodył A. Evidence for horizontal gene transfer from bacteroidetes bacteria to dinoflagellate minicircles. Mol Biol Evol 2012; 29:887 - 92; http://dx.doi.org/10.1093/molbev/msr276; PMID: 22075114
- Chan CX, Zäuner S, Wheeler G, Grossman AR, Prochnik SE, Blouin NA, Zhuang Y, Benning C, Berg GM, Yarish C, et al. Analysis of Porphyra membrane transporters demonstrates gene transfer among photosynthetic eukaryotes and numerous sodium-coupled transport systems. Plant Physiol 2012; 158:2001 - 12; http://dx.doi.org/10.1104/pp.112.193896; PMID: 22337920
- Chan CX, Yang EC, Banerjee T, Yoon HS, Martone PT, Estevez JM, Bhattacharya D. Red and green algal monophyly and extensive gene sharing found in a rich repertoire of red algal genes. Curr Biol 2011; 21:328 - 33; http://dx.doi.org/10.1016/j.cub.2011.01.037; PMID: 21315598
- Huang J, Gogarten JP. Did an ancient chlamydial endosymbiosis facilitate the establishment of primary plastids?. Genome Biol 2007; 8:R99; http://dx.doi.org/10.1186/gb-2007-8-6-r99; PMID: 17547748
- Qiu H, Price DC, Weber APM, Facchinelli F, Yoon HS, Bhattacharya D. Assessing the bacterial contribution to the plastid proteome. Trends Plant Sci 2013; 18:680 - 7; http://dx.doi.org/10.1016/j.tplants.2013.09.007; PMID: 24139901
- Moustafa A, Beszteri B, Maier UG, Bowler C, Valentin K, Bhattacharya D. Genomic footprints of a cryptic plastid endosymbiosis in diatoms. Science 2009; 324:1724 - 6; http://dx.doi.org/10.1126/science.1172983; PMID: 19556510
- Dorrell RG, Smith AG. Do red and green make brown?: perspectives on plastid acquisitions within chromalveolates. Eukaryot Cell 2011; 10:856 - 68; http://dx.doi.org/10.1128/EC.00326-10; PMID: 21622904
- Burki F, Flegontov P, Oborník M, Cihlár J, Pain A, Lukeš J, Keeling PJ. Re-evaluating the green versus red signal in eukaryotes with secondary plastid of red algal origin. Genome Biol Evol 2012; 4:626 - 35; http://dx.doi.org/10.1093/gbe/evs049; PMID: 22593553
- Deschamps P, Moreira D. Reevaluating the green contribution to diatom genomes. Genome Biol Evol 2012; 4:683 - 8; http://dx.doi.org/10.1093/gbe/evs053; PMID: 22684208
- Chan CX, Reyes-Prieto A, Bhattacharya D. Red and green algal origin of diatom membrane transporters: insights into environmental adaptation and cell evolution. PLoS One 2011; 6:e29138; http://dx.doi.org/10.1371/journal.pone.0029138; PMID: 22195008
- Curtis BA, Tanifuji G, Burki F, Gruber A, Irimia M, Maruyama S, Arias MC, Ball SG, Gile GH, Hirakawa Y, et al. Algal genomes reveal evolutionary mosaicism and the fate of nucleomorphs. Nature 2012; 492:59 - 65; http://dx.doi.org/10.1038/nature11681; PMID: 23201678
- Frommolt R, Werner S, Paulsen H, Goss R, Wilhelm C, Zauner S, Maier UG, Grossman AR, Bhattacharya D, Lohr M. Ancient recruitment by chromists of green algal genes encoding enzymes for carotenoid biosynthesis. Mol Biol Evol 2008; 25:2653 - 67; http://dx.doi.org/10.1093/molbev/msn206; PMID: 18799712
- Peers G, Truong TB, Ostendorf E, Busch A, Elrad D, Grossman AR, Hippler M, Niyogi KK. An ancient light-harvesting protein is critical for the regulation of algal photosynthesis. Nature 2009; 462:518 - 21; http://dx.doi.org/10.1038/nature08587; PMID: 19940928
- Chan CX, Baglivi FL, Jenkins CE, Bhattacharya D. Foreign gene recruitment to the fatty acid biosynthesis pathway in diatoms. Mob Genet Elements 2013; 3:e27313; http://dx.doi.org/10.4161/mge.27313
- Gogarten JP, Townsend JP. Horizontal gene transfer, genome innovation and evolution. Nat Rev Microbiol 2005; 3:679 - 87; http://dx.doi.org/10.1038/nrmicro1204; PMID: 16138096
- Lawrence JG, Ochman H. Amelioration of bacterial genomes: rates of change and exchange. J Mol Evol 1997; 44:383 - 97; http://dx.doi.org/10.1007/PL00006158; PMID: 9089078
- LaJeunesse TC, Lambert G, Andersen RA, Coffroth MA, Galbraith DW. Symbiodinium (Pyrrhophyta) genome sizes (DNA content) are smallest among dinoflagellates. J Phycol 2005; 41:880 - 6; http://dx.doi.org/10.1111/j.0022-3646.2005.04231.x
- Hackett JD, Bhattacharya D. The genomes of dinoflagellates. In: Katz LA, Bhattacharya D, eds. Genomics and Evolution of Microbial Eukaryotes. New York: Oxford University Press, 2008:48-63.
- Lin S. Genomic understanding of dinoflagellates. Res Microbiol 2011; 162:551 - 69; http://dx.doi.org/10.1016/j.resmic.2011.04.006; PMID: 21514379
- Rosic N, Hoegh-Guldberg O. A method for extracting a high-quality RNA from Symbiodinium sp. J Appl Phycol 2010; 22:139 - 46; http://dx.doi.org/10.1007/s10811-009-9433-x
- Sim MC, Ho CL, Phang SM. A simple and effective method for RNA isolation and cDNA library construction from the brown seaweed Sargassum polycystum (Fucales, Phaeophyceae). J Appl Phycol 2013; 25:1277 - 85; http://dx.doi.org/10.1007/s10811-013-9980-z
- Philippe H, Brinkmann H, Lavrov DV, Littlewood DTJ, Manuel M, Wörheide G, Baurain D. Resolving difficult phylogenetic questions: why more sequences are not enough. PLoS Biol 2011; 9:e1000602; http://dx.doi.org/10.1371/journal.pbio.1000602; PMID: 21423652
- Stiller JW. Experimental design and statistical rigor in phylogenomics of horizontal and endosymbiotic gene transfer. BMC Evol Biol 2011; 11:259; http://dx.doi.org/10.1186/1471-2148-11-259; PMID: 21923904
- Chan CX, Ragan MA. Next-generation phylogenomics. Biol Direct 2013; 8:3; http://dx.doi.org/10.1186/1745-6150-8-3; PMID: 23339707
- Höhl M, Ragan MA. Is multiple-sequence alignment required for accurate inference of phylogeny?. Syst Biol 2007; 56:206 - 21; http://dx.doi.org/10.1080/10635150701294741; PMID: 17454975
- Comin M, Verzotto D. Alignment-free phylogeny of whole genomes using underlying subwords. Algorithms Mol Biol 2012; 7:34; http://dx.doi.org/10.1186/1748-7188-7-34; PMID: 23216990
- Domazet-Lošo M, Haubold B. Alignment-free detection of horizontal gene transfer between closely related bacterial genomes. Mob Genet Elements 2011; 1:230 - 5; http://dx.doi.org/10.4161/mge.1.3.18065; PMID: 22312592