Abstract
Rubinstein–Taybi Syndrome (RTS), where the transcriptional co-activator and histone acetyltransferase CBP is mutated and haploinsufficient, is often associated with epilepsy, a disorder that is frequently due to perturbations in the generation of GABAergic interneurons and/or the inhibitory neurotransmitter GABA. Hereby, Tsui et al., recently published in Developmental Biology, asked whether CBP was necessary for the appropriate genesis and differentiation of interneurons in the murine forebrain. This paper defined multiple roles of CBP during forebrain interneuron development. In particular, CBP not only acts as a pro-differentiation factor to enhance the differentiation of ventral forebrain precursors to interneurons, but also modulates the maturation of interneurons by promoting acquisition of a GABAergic interneuron phenotype in the newborn neurons. Thus, deficits in interneuron development caused by CBP haploinsufficiency provide a potential explanation for the epilepsy seen in individuals with RTS.
Introduction
CBP (CREB binding protein), a transcriptional co-activator and histone acetyltransferase (HAT), is a major cause of Rubinstein–Taybi syndrome (RTS), when haploinsufficient. RTS is a human genetic neurodevelopmental disorder associated with cognitive dysfunction.Citation1 Because of this link to a human genetic disorder, CBP has received significant attention with regard to its potential functional roles in brain development and cognition. For example, previous work has shown that CBP HAT activity plays a critical role in memory consolidation by regulating hippocampal synaptic plasticity.Citation2,Citation3 Moreover, our previous work demonstrated that aberrant neural development caused by CBP haploinsufficiency might cause the early intellectual disability observed in RTS.Citation4 Specifically, we showed that CBP promoted differentiation of embryonic cortical precursors by enhancing histone acetylation at the promoter regions of glial and neuronal genes.Citation4 Thus, when CBP was haploinsufficient in a mouse model of RTS, this decreased the genesis of both glia and cortical excitatory projection neurons in the developing forebrain, and led to behavioral perturbations during early postnatal life.Citation4
CBP is a Pro-Differentiation Factor for MGE Precursor Development
In Tsui et al.,Citation5 we extended our studies to ventral forebrain precursors with regard to a potential functional role for CBP during brain development. Murine ventral forebrain precursors within the medial ganglionic eminence (MGE) region generate the large majority of interneurons which migrate from the MGE along the superficial and deep tangential migratory streams to enter the cortex.Citation6,Citation7 MGE precursors also produce the first wave of oligodendrocyte progenitors that populate the entire embryonic telencephalon including the cerebral cortex.Citation8 Eventually, the MGE-derived oligodendrocyte population is eliminated during postnatal life and replaced by oligodendrocytes generated by precursors within the cortex itself.Citation8 In contrast, astrocytes are not generated in the developing ventral forebrain in vivoCitation9 although the ventral forebrain precursors, cultured as floating neurospheres, have the capacity to differentiate into astrocytes in vitro.Citation10 In Tsui et al.,Citation5 we established a MGE-derived ventral forebrain precursor culture system, which mimics the ventral forebrain development in vivo, generating interneurons first and oligodendrocytes second, with minimal genesis of astrocytes. We demonstrate that CBP acts as a pro-differentiation factor to drive the differentiation of MGE precursors into interneurons and oligodendrocytes. In particular, CBP knockdown in MGE precursors significantly increased the proportion of MGE precursor cells while decreasing the genesis of newborn interneurons and oligodendrocytes without affecting MGE precursor proliferation or apoptosis. Hence, our results support and extend our previous studyCitation4 demonstrating a similar role of CBP in embryonic cortical precursors.
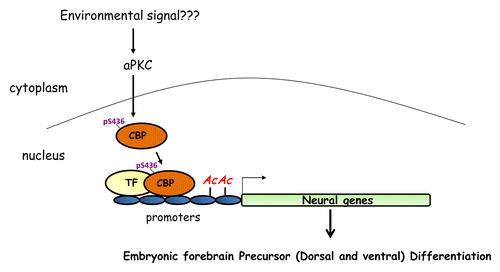
How does CBP regulate the differentiation of MGE precursors? In our previous study,Citation4 chromatin immunoprecipitation (ChIP) analyses of the embryonic and postnatal cerebral cortex showed that CBP regulated histone acetylation of glial and neuronal gene promoters to trigger embryonic cortical precursor differentiation. We predict that CBP would regulate MGE precursor differentiation in the same manner () since the phenotypic changes after CBP knockdown in MGE precursors are very similar to those observed following CBP knockdown in embryonic cortical precursors. Additionally, our previous reportCitation4 showed that Serine (S) 436 in CBP is phosphorylated by atypical protein kinase C (aPKC) zeta and that this is a key epigenetic switch that makes embryonic cortical precursors competent to differentiate. It will be of great interest to study the role of CBP S436 phosphorylation during MGE precursor development and to understand how CBP S436 phosphorylation regulates the epigenetic status of MGE precursors (). The available CBPS436A knock-in mouse strainCitation11 provides a useful tool for us to delineate the aPKC-CBP pathway in vivo during ventral forebrain development.
Figure 1. A schematic model describing how CBP promotes embryonic (both dorsal and ventral) forebrain precursor differentiation. S436 phosphorylation in CBP by aPKC recruits CBP to the promoter regions of neural specific genes in both embryonic dorsal and ventral forebrain precursors to enhance histone acetylation and promote precursor differentiation. TF, transcription factor; aPKC, atypical protein kinase C.
CBP Promotes Acquisition of an Interneuron Phenotype during Ventral Forebrain Development
Interestingly, in Tsui et al.,Citation5 we show that acquisition of a GABAergic interneuron phenotype in newborn neurons generated from MGE precursors lags several days after the first neuronal marker, βIII tubulin, is expressed in the cells. Thus, GABAergic interneuron development is composed of two consecutive steps, initial neurogenesis and interneuron maturation. Dysfunction in the generation of GABAergic interneurons and/or the inhibitory neurotransmitter GABA often leads to seizures/epilepsy, which is a symptom frequently associated with RTS.Citation12 Here, we demonstrate that CBP is not only required for initial genesis of newborn neurons from MGE precursors, but that it is also essential for GABAergic interneuron maturation (acquisition of an interneuron phenotype in newborn neurons). This provides, at least in part, a potential explanation for the epilepsy associated with RTS. Although it remains unknown how CBP regulates these two steps of interneuron development, we show that CBP HAT activity is required for the genesis of GAD67 (a GABA neurotransmitter synthesis enzyme) expressing interneurons. We predict that CBP acts as a transcriptional coactivator that binds to the promoter regions of neuronal-specific genes like Tα-tubulin,Citation4 and GABAergic interneuron-specific genes like GAD67,Citation13 to epigenetically regulate their expression in MGE precursors and newborn neurons. Future ChIP-sequencing of MGE tissue with anti-CBP and anti-H3K14 antibodies will lead us to a definitive landscape of CBP abundance on genomic regulatory regions of interneuron and interneuron progenitor genes. This will add to our mechanistic understanding of the critical epigenetic factor during ventral forebrain development.
CBP: A Regulator of Adult Neurogenesis and Maturation
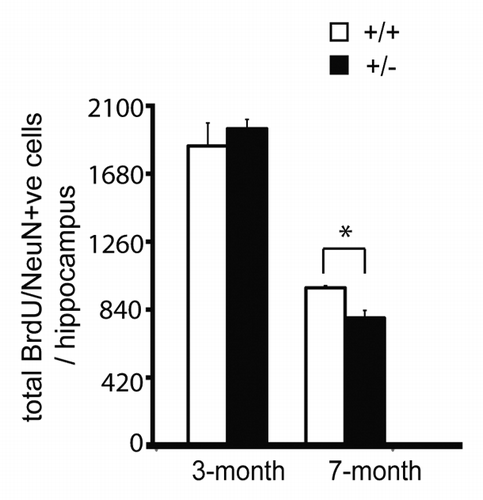
The two adult neurogenic regions, the subventricular zone of the lateral ventricles and the subgranular zone of the hippocampal dentate gyrus, contain astrocyte-like neural stem cells (NSCs) that are thought to derive from embryonic radial glial precursor cells (RGs).Citation14,Citation15 Tsui et al.,Citation5 together with our previous study,Citation4 demonstrate that CBP is essential for the appropriate genesis of both cortical excitatory projection neurons and interneurons from embryonic RGs in the cortex and MGE, respectively. Because of the embryonic origins of adult NSCs, we posit that CBP is a key regulator of adult neurogenesis and maturation. This argument is supported by recent studies showing that CBP is required to regulate adult neurogenesis induced by environmental enrichment and metformin treatment.Citation16,Citation17 Our recent study also showed that CBP regulated adult hippocampal neurogenesis in an age-dependent manner (, modified from Wang et al.Citation17). A thorough understanding of the functional roles of CBP in the regulation of adult neurogenesis and neuronal maturation will be of great interest to our future work as it would provide a novel strategy to enhance neural regeneration with high fidelity by recapitulating NSC-mediated developmental processes.Citation18
Figure 2. CBP haploinsufficiency causes decreased hippocampal neurogenesis in an age-dependent manner. CBP+/+ and CBP+/− mice at the ages of 3 mo and 7 mo were injected with BrdU and sacrificed 12 d later for quantifying total number of BrdU and NeuN positive cells in coronal sections of the hippocampal dentate gyrus. Quantitative analysis of the total number of BrdU/NeuN-positive cells in the hippocampi from CBP+/+ and CBP+/− (3-mo- and 7-mo-old). *P < 0.05 (n = 4–6 to each group). Error bars denote SEM.
Summary
Tsui et al.Citation5 for the first time defines multiple roles for CBP during GABAergic interneuron development in the embryonic ventral forebrain. CBP, acting as a pro-differentiation factor and maturation regulator, ensures the genesis of appropriate numbers of GABAergic interneurons during embryonic and early postnatal brain development. When CBP is haploinsufficient in a mouse model of RTS, it causes deficits in forebrain interneuron development in early postnatal life, providing an explanation for the epilepsy phenotype associated with human RTS patients. The essential role of CBP during embryonic cortex and ventral forebrain development prompts us to explore its functional role in the regulation of adult neurogenesis and maturation and its therapeutic potential for neural regeneration.
| Abbreviations: | ||
| CBP | = | CREB binding protein |
| RTS | = | Rubinstein–Taybi Syndrome |
| HAT | = | histone acetyltransferase |
| MGE | = | medial ganglionic eminence |
| ChIP | = | chromatin immunoprecipitation |
| NSC | = | neural stem cell |
| RG | = | radial glial precursor cell |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Wiley S, Swayne S, Rubinstein JH, Lanphear NE, Stevens CA. Rubinstein-Taybi syndrome medical guidelines. Am J Med Genet A 2003; 119A:101 - 10; http://dx.doi.org/10.1002/ajmg.a.10009; PMID: 12749047
- Alarcón JM, Malleret G, Touzani K, Vronskaya S, Ishii S, Kandel ER, Barco A. Chromatin acetylation, memory, and LTP are impaired in CBP+/- mice: a model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron 2004; 42:947 - 59; http://dx.doi.org/10.1016/j.neuron.2004.05.021; PMID: 15207239
- Korzus E, Rosenfeld MG, Mayford M. CBP histone acetyltransferase activity is a critical component of memory consolidation. Neuron 2004; 42:961 - 72; http://dx.doi.org/10.1016/j.neuron.2004.06.002; PMID: 15207240
- Wang J, Weaver IC, Gauthier-Fisher A, Wang H, He L, Yeomans J, Wondisford F, Kaplan DR, Miller FD. CBP histone acetyltransferase activity regulates embryonic neural differentiation in the normal and Rubinstein-Taybi syndrome brain. Dev Cell 2010; 18:114 - 25; http://dx.doi.org/10.1016/j.devcel.2009.10.023; PMID: 20152182
- Tsui D, Voronova A, Gallagher D, Kaplan DR, Miller FD, Wang J. CBP regulates the differentiation of interneurons from ventral forebrain neural precursors during murine development. Dev Biol 2014; 385:230 - 41; http://dx.doi.org/10.1016/j.ydbio.2013.11.005; PMID: 24247009
- Lavdas AA, Grigoriou M, Pachnis V, Parnavelas JG. The medial ganglionic eminence gives rise to a population of early neurons in the developing cerebral cortex. J Neurosci 1999; 19:7881 - 8; PMID: 10479690
- Xu Q, Tam M, Anderson SA. Fate mapping Nkx2.1-lineage cells in the mouse telencephalon. J Comp Neurol 2008; 506:16 - 29; http://dx.doi.org/10.1002/cne.21529; PMID: 17990269
- Kessaris N, Fogarty M, Iannarelli P, Grist M, Wegner M, Richardson WD. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat Neurosci 2006; 9:173 - 9; http://dx.doi.org/10.1038/nn1620; PMID: 16388308
- Rowitch DH. Glial specification in the vertebrate neural tube. Nat Rev Neurosci 2004; 5:409 - 19; http://dx.doi.org/10.1038/nrn1389; PMID: 15100723
- Shakèd M, Weissmüller K, Svoboda H, Hortschansky P, Nishino N, Wölfl S, Tucker KL. Histone deacetylases control neurogenesis in embryonic brain by inhibition of BMP2/4 signaling. PLoS One 2008; 3:e2668; http://dx.doi.org/10.1371/journal.pone.0002668; PMID: 18628975
- He L, Sabet A, Djedjos S, Miller R, Sun X, Hussain MA, Radovick S, Wondisford FE. Metformin and insulin suppress hepatic gluconeogenesis through phosphorylation of CREB binding protein. Cell 2009; 137:635 - 46; http://dx.doi.org/10.1016/j.cell.2009.03.016; PMID: 19450513
- Cantani A, Gagliesi D. Rubinstein-Taybi syndrome. Review of 732 cases and analysis of the typical traits. Eur Rev Med Pharmacol Sci 1998; 2:81 - 7; PMID: 10229563
- Asahara H, Santoso B, Guzman E, Du K, Cole PA, Davidson I, Montminy M. Chromatin-dependent cooperativity between constitutive and inducible activation domains in CREB. Mol Cell Biol 2001; 21:7892 - 900; http://dx.doi.org/10.1128/MCB.21.23.7892-7900.2001; PMID: 11689682
- Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci 2009; 32:149 - 84; http://dx.doi.org/10.1146/annurev.neuro.051508.135600; PMID: 19555289
- Kempermann G, Jessberger S, Steiner B, Kronenberg G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci 2004; 27:447 - 52; http://dx.doi.org/10.1016/j.tins.2004.05.013; PMID: 15271491
- Lopez-Atalaya JP, Ciccarelli A, Viosca J, Valor LM, Jimenez-Minchan M, Canals S, Giustetto M, Barco A. CBP is required for environmental enrichment-induced neurogenesis and cognitive enhancement. EMBO J 2011; 30:4287 - 98; http://dx.doi.org/10.1038/emboj.2011.299; PMID: 21847097
- Wang J, Gallagher D, DeVito LM, Cancino GI, Tsui D, He L, Keller GM, Frankland PW, Kaplan DR, Miller FD. Metformin activates an atypical PKC-CBP pathway to promote neurogenesis and enhance spatial memory formation. Cell Stem Cell 2012; 11:23 - 35; http://dx.doi.org/10.1016/j.stem.2012.03.016; PMID: 22770240
- Qureshi IA, Mehler MF. The emerging role of epigenetics in stroke: III. Neural stem cell biology and regenerative medicine. Arch Neurol 2011; 68:294 - 302; http://dx.doi.org/10.1001/archneurol.2011.6; PMID: 21403016
