Abstract
Withdrawal of differentiating neurons from the ventricle of the neural tube is a highly regulated process that is critical for the formation of normal tissue architecture and neural circuitry. We have recently reported a novel cell-biological event that mediates this process and involves local abscission of the apical cell-compartment as cells become neurons. Apical abscission takes place as adherens junctions are lost, depends on actinomyosin contractility, results in loss of apical cell polarity as well as dis-assembly of the primary cilium, and allows cell cycle exit. The molecular mechanisms mediating the series of steps underlying apical abscission are complex and inter-linked and open up many new questions. In this commentary we discuss how these mechanisms may operate and the functions of apical abscission during neurogenesis, in disease, and in other cell biological processes that involve withdrawal of cells from epithelia.
Introduction
Becoming a neuron is a complex process. It involves not only exit from the cell cycle but also dramatic change in cell shape and movement. Neurons are generated in the proliferative ventricular layer, located at the lumen of the neural tube and they leave this region to form the mantle layer or cortical layers within the developing brain. A prerequisite for this migration is the detachment of the newborn neuron from the luminal or apical surface. Failure to do so leads to the ectopic accumulation of neurons, which can result in human periventricular heterotopia, a syndrome that includes reduced brain size, epilepsy and dyslexia.Citation1
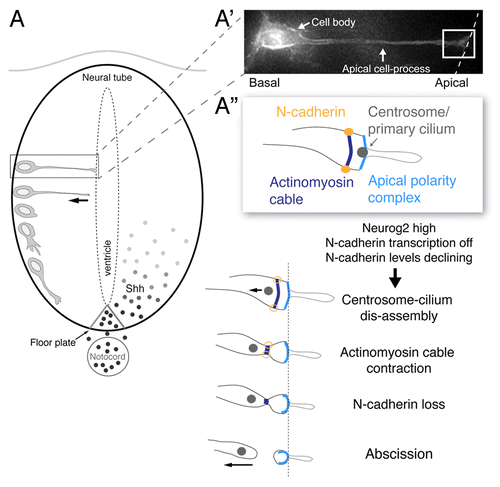
Cells about to undergo neuronal differentiation have a very particular morphology: a basally located cell body and a long thin cellular process with an apical endfoot that contributes to the ventricular/apical surface ().Citation2 These cells exhibit apico-basal polarity, characterized by localization of the Par complex proteins (Par3, Par6, and aPKC) to the apical-most cell membrane. Such cells also possess an apically positioned primary cilium, which is required to transduce mitogenic Sonic hedgehog (Shh) signals emanating from the floor plate of the neural tube.Citation3 These cells also share sub-apical adherens junctions with neighboring cells that interact with an intracellular contractile actinomyosin cable that transmits forces to the cell cortex. An initial step in the detachment from the ventricle involves breakdown of these inter-cellular connections involving loss of the adherens junction component N-cadherin ().Citation4-Citation6 Surprisingly, we observed that following N-cadherin loss the prospective neuron undergoes local abscission of its apical membrane, resulting in loss of apical cell polarity. We further demonstrated that this apical abscission event is driven by constriction of the sub-apical actinomyosin cable. During apical abscission, we observed a dis-assembly of the centrosome-primary cilium complex, causing the centrosome to be retained by the withdrawing neuron while the ciliary membrane is left behind in the abscised particle. This suggests that the newborn neuron is now incapable of signaling via Shh; and this, in turn facilitates cell cycle exit. Consistent with this we found that blocking abscission by N-Cadherin mis-expression inhibited onset of the cell cycle exit gene p27.
Figure 1. Steps underlying apical abscission in the neural tube. (A) Schematic of sequence of events taking place as a cell differentiates into a neuron in the early neural tube/spinal cord, beginning with the withdrawal of the apical cell-process from the ventricular surface and subsequent production of a growth cone and primary axon extension. Sonic hedgehog (Shh) signals from the notochord/floor plate promote proliferation and pattern in the spinal cord and their transduction requires an intact primary cilium; (A’) A cell poised to undergo neuronal differentiation, characterized by a basally located cell body and attachment to the apical surface through an elongated apical cell-process. (A”) Schematic of key sub-cellular structures in the apical endfoot of the prospective neuron (white boxed region in A’) (including apical complex containing apical membrane, the primary cilium, cadherin-containing adherens junctions and associated actinomyosin cable) and sequential changes leading to apical abscission following loss of N-cadherin.
Molecular Mechanisms Regulating N-Cadherin Loss
Ectopic maintenance of N-Cadherin also blocked centrosome-cilium dis-assembly as well as abscission, while mis-expression of the proneural factor Neurog2 was able to rescue this by driving depletion of ectopically expressed N-cadherin protein. The molecular mechanisms that deplete N-cadherin from the adherens junction remain unclear, although our observations place Neurog2 upstream of this process. Dynamic instability is a key feature of adherens junctions in vivo and in vitro, and this is mediated by clathrin-dependent endocytic turnover of cadherins. Endocytic turnover is negatively regulated by the cadherin-binding partner p120 cateninCitation7-Citation9 and the adherens junction-associated protein Numb.Citation10 Our observations further support that turnover of N-cadherin is a tightly controlled process during neurogenesis and raise the possibility that attenuation of N-cadherin recycling by altered levels of p120 catenin and/or Numb, resulting in less robust cell–cell junctions and increased actinomyosin contractility may regulate progression of apical abscission.
Similarities between Apical Abscission and Cytokinesis
Apical abscission is characterized by a local increase in sub-apical actin density and myosin activity (). This is remarkably similar to cytokinesis where a contractile actinomyosin ring cleaves the cell in to twoCitation11 and one possibility is that apical abscission utilizes some cytokinesis machinery. Unlike cytokinesis, however, apical abscission is an asymmetric process, allocating a single centrosome (and nucleus) on one side of the abscission site and the apical and ciliary membrane on the other. While cytokinesis requires de-novo formation of a contractile actinomyosin cable,Citation12-Citation15 neuroepithelial cells already possess a sub-apical actinomyosin cable that mediates apical constriction.Citation16-Citation18 The central spindle, consisting of stable antiparallel microtubules,Citation19 marks the site of the cytokinetic furrow and stimulates actinomyosin contractility during cytokinesis.Citation20 Future studies will determine if formation of a similar microtubule array at the site of apical abscission is required for actinomyosin constriction to proceed. Furthermore, super-resolution structured illumination microscopy revealed a thin membranous connection between the withdrawing apical cell-process and the abscising particle similar to the cytokinetic midbody, suggesting that a final membrane scission event is involved at completion of apical abscission. The molecular machinery that mediates this is likely to be similar to cytokinetic membrane scission, as this is highly evolutionarily conserved.Citation21,Citation22
Molecular Mechanisms of Primary Cilium Disassembly
During mitosis, the primary cilium is resorbed or partially internalized.Citation23,Citation24 This is distinct from apical abscission, as here the ciliary membrane is released along with the abscised membrane (). We observed that neurons poised to abscise exhibit accumulation of the hedgehog transducer Smoothened and its pathway effector Gli2 in their primary cilia, which is indicative of cells responding to hedgehog signals;Citation25 apical abscission may therefore effectively curtail such mitogenic signaling, thereby promoting cell cycle exit. The only other example of regulated cilium shedding has been observed during deflagellation in the blue-green alga Chlamydomonas.Citation26 This is mediated by microtubule severing proteins and results in shedding of the whole axonemal structure.Citation27 It will be important to determine if apical abscission also involves severance of the axoneme and whether/how this may be linked to actinomyosin contractility through, for example, the formation of antiparallel microtubule arrays.
Apical Abscission as a Mechanism for Reconfiguring the Newborn Neuron
Apical abscission thus appears to be a pivotal moment in the neuronal differentiation program as it not only mediates acute loss of apical cell polarity, but also transiently releases the cell from the influence of extracellular signals mediated by the primary cilium (). This may provide a critical transition period during which the newborn neuron re-polarizes and re-organizes its cytoskeleton to establish the site of the nascent axon.Citation6,Citation28 The release of the centrosome from the primary cilium and the discarding of apical complex containing membrane may be necessary for this step, allowing re-deployment of cell polarity machinery and re-positioning of the centrosome, which is thought to influence this process.Citation29,Citation30 The centrosome will also be required for later signaling through a new cilium, which is regenerated in the differentiating neuron. Indeed, this shedding and replenishing of the ciliary membrane may mediate change in the cell’s response to signals such as Shh, which, although initially mitogenic in the neural tube, is later re-deployed as an axon guidance molecule.Citation31 Neuronal primary cilia also play further roles, for example, in the regulation of cortical interneuron migration.Citation32
Maintenance of Tissue Homeostasis
Abscising the apical membrane and leaving this, at least initially, at the apical surface may allow neighboring cells to adjust to this loss of cell–cell contact and so help to maintain epithelial tissue integrity. Furthermore, Notch and its ligands Dll1 and Jagged1 are enriched at the apical end feet of neural progenitor cells where they mediate lateral inhibitionCitation33-Citation36 and interact with ZO-1, a constituent of the adherens junctions.Citation35 This suggests that interactions at the apical domains of neuroepithelial cells play a key role in regulating neuronal differentiation; when a newborn neuron undergoes apical abscission it may relieve neighboring neural progenitors from Notch signalling, allowing these cells in turn the opportunity to become neurons. It is also possible that persisting discarded apical membrane, enriched for Notch ligands, at least briefly prevents neural progenitors from differentiating into neurons.
Apical Abscission and Human Disease
Human periventricular heterotopia involves failure of neurons to leave the ventricular surface and is anatomically characterized by nodular structures containing these ectopically located neurons. A major cause is mutation in the actin-binding protein Filamin-1 (an X-linked dominant locus), which cross-links actin filaments and creates the meshwork required for actin–myosin interaction.Citation37 Future studies will determine if defective apical abscission, which we show depends on actin–myosin contractility, contributes to this phenotype.
Finally, loss of apical complex proteins and disruption of adherens junctions also characterizes cells undergoing an epithelial to mesenchymal transition (EMT). Indeed, transcription factors (Scratch1/2) belonging to the Snail super-family have been shown to attenuate E-cadherin expression in prospective cortical neuronsCitation38 indicating that elements of the EMT machinery also operate during neuronal detachment. However, knockdown of apical complex proteins such as Par3 can increase the probability of tumor cell metastasis even without triggering a full EMT.Citation39 Some cancers also exhibit cilia loss, which depending on the underlying mutation, can augment or attenuate disease progression.Citation40,Citation41 The mechanism underlying cilia loss in cancer cells is unknown and it will be important to determine whether this and progression to metastasis involve apical abscission events. It is also possible that apical abscission take places in other regions of the embryo undergoing EMT, such as the neural crest and during gastrulation as epiblast cells ingress through the primitive streak and acquire a mesodermal fate.Citation42 These important next steps will establish the generality of apical abscission as mechanism for cell state change.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors were supported by a Wellcome Trust Investigator Award WT102817AIA.
References
- SheenVL. Periventricular Heterotopia: Shuttling of Proteins through Vesicles and Actin in Cortical Development and Disease. Scientifica (Cairo)2012; 2012:480129; http://dx.doi.org/10.6064/2012/480129; PMID: 24278701
- DasRM, StoreyKG. Apical abscission alters cell polarity and dismantles the primary cilium during neurogenesis. Science2014; 343:200 - 4; http://dx.doi.org/10.1126/science.1247521; PMID: 24408437
- LouviA, GroveEA. Cilia in the CNS: the quiet organelle claims center stage. Neuron2011; 69:1046 - 60; http://dx.doi.org/10.1016/j.neuron.2011.03.002; PMID: 21435552
- WongGKW, BaudetM-L, NordenC, LeungL, HarrisWA. Slit1b-Robo3 signaling and N-cadherin regulate apical process retraction in developing retinal ganglion cells. J Neurosci2012; 32:223 - 8; http://dx.doi.org/10.1523/JNEUROSCI.2596-11.2012; PMID: 22219284
- ShovalI, LudwigA, KalcheimC. Antagonistic roles of full-length N-cadherin and its soluble BMP cleavage product in neural crest delamination. Development2007; 134:491 - 501; http://dx.doi.org/10.1242/dev.02742; PMID: 17185320
- RoussoDL, PearsonCA, GaberZB, MiquelajaureguiA, LiS, Portera-CailliauC, MorriseyEE, NovitchBG. Foxp-mediated suppression of N-cadherin regulates neuroepithelial character and progenitor maintenance in the CNS. Neuron2012; 74:314 - 30; http://dx.doi.org/10.1016/j.neuron.2012.02.024; PMID: 22542185
- de BecoS, GueudryC, AmblardF, CoscoyS. Endocytosis is required for E-cadherin redistribution at mature adherens junctions. Proc Natl Acad Sci U S A2009; 106:7010 - 5; http://dx.doi.org/10.1073/pnas.0811253106; PMID: 19372377
- FujitaY, KrauseG, ScheffnerM, ZechnerD, LeddyHEM, BehrensJ, SommerT, BirchmeierW. Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat Cell Biol2002; 4:222 - 31; http://dx.doi.org/10.1038/ncb758; PMID: 11836526
- DavisMA, IretonRC, ReynoldsAB. A core function for p120-catenin in cadherin turnover. J Cell Biol2003; 163:525 - 34; http://dx.doi.org/10.1083/jcb.200307111; PMID: 14610055
- RasinM-R, GazulaV-R, BreunigJJ, KwanKY, JohnsonMB, Liu-ChenS, LiH-S, JanLY, JanY-N, RakicP, et al. Numb and Numbl are required for maintenance of cadherin-based adhesion and polarity of neural progenitors. Nat Neurosci2007; 10:819 - 27; http://dx.doi.org/10.1038/nn1924; PMID: 17589506
- FededaJP, GerlichDW. Molecular control of animal cell cytokinesis. Nat Cell Biol2012; 14:440 - 7; http://dx.doi.org/10.1038/ncb2482; PMID: 22552143
- MurthyK, WadsworthP. Myosin-II-dependent localization and dynamics of F-actin during cytokinesis. Curr Biol2005; 15:724 - 31; http://dx.doi.org/10.1016/j.cub.2005.02.055; PMID: 15854904
- UeharaR, GoshimaG, MabuchiI, ValeRD, SpudichJA, GriffisER. Determinants of myosin II cortical localization during cytokinesis. Curr Biol2010; 20:1080 - 5; http://dx.doi.org/10.1016/j.cub.2010.04.058; PMID: 20541410
- YumuraS, UedaM, SakoY, Kitanishi-YumuraT, YanagidaT. Multiple mechanisms for accumulation of myosin II filaments at the equator during cytokinesis. Traffic2008; 9:2089 - 99; http://dx.doi.org/10.1111/j.1600-0854.2008.00837.x; PMID: 18939956
- ZhouM, WangY-L. Distinct pathways for the early recruitment of myosin II and actin to the cytokinetic furrow. Mol Biol Cell2008; 19:318 - 26; http://dx.doi.org/10.1091/mbc.E07-08-0783; PMID: 17959823
- HaigoSL, HildebrandJD, HarlandRM, WallingfordJB. Shroom induces apical constriction and is required for hingepoint formation during neural tube closure. Curr Biol2003; 13:2125 - 37; http://dx.doi.org/10.1016/j.cub.2003.11.054; PMID: 14680628
- IshiuchiT, TakeichiM. Willin and Par3 cooperatively regulate epithelial apical constriction through aPKC-mediated ROCK phosphorylation. Nat Cell Biol2011; 13:860 - 6; http://dx.doi.org/10.1038/ncb2274; PMID: 21685893
- NishimuraT, TakeichiM. Shroom3-mediated recruitment of Rho kinases to the apical cell junctions regulates epithelial and neuroepithelial planar remodeling. Development2008; 135:1493 - 502; http://dx.doi.org/10.1242/dev.019646; PMID: 18339671
- GlotzerM. The 3Ms of central spindle assembly: microtubules, motors and MAPs. Nat Rev Mol Cell Biol2009; 10:9 - 20; http://dx.doi.org/10.1038/nrm2609; PMID: 19197328
- FoeVE, von DassowG. Stable and dynamic microtubules coordinately shape the myosin activation zone during cytokinetic furrow formation. J Cell Biol2008; 183:457 - 70; http://dx.doi.org/10.1083/jcb.200807128; PMID: 18955555
- NetoH, GouldGW. The regulation of abscission by multi-protein complexes. J Cell Sci2011; 124:3199 - 207; http://dx.doi.org/10.1242/jcs.083949; PMID: 21940792
- EliaN, SougratR, SpurlinTA, HurleyJH, Lippincott-SchwartzJ. Dynamics of endosomal sorting complex required for transport (ESCRT) machinery during cytokinesis and its role in abscission. Proc Natl Acad Sci U S A2011; 108:4846 - 51; http://dx.doi.org/10.1073/pnas.1102714108; PMID: 21383202
- ParidaenJTML, Wilsch-BräuningerM, HuttnerWB. Asymmetric inheritance of centrosome-associated primary cilium membrane directs ciliogenesis after cell division. Cell2013; 155:333 - 44; http://dx.doi.org/10.1016/j.cell.2013.08.060; PMID: 24120134
- KimS, TsiokasL. Cilia and cell cycle re-entry: more than a coincidence. Cell Cycle2011; 10:2683 - 90; http://dx.doi.org/10.4161/cc.10.16.17009; PMID: 21814045
- KimJ, KatoM, BeachyPA. Gli2 trafficking links Hedgehog-dependent activation of Smoothened in the primary cilium to transcriptional activation in the nucleus. Proc Natl Acad Sci U S A2009; 106:21666 - 71; http://dx.doi.org/10.1073/pnas.0912180106; PMID: 19996169
- Quarmby LM. Cellular Deflagellation. Int Rev Cytol: Academic Press, 2004:47-91.
- LohretTA, McNallyFJ, QuarmbyLM. A role for katanin-mediated axonemal severing during Chlamydomonas deflagellation. Mol Biol Cell1998; 9:1195 - 207; http://dx.doi.org/10.1091/mbc.9.5.1195; PMID: 9571249
- NoctorSC, Martínez-CerdeñoV, IvicL, KriegsteinAR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci2004; 7:136 - 44; http://dx.doi.org/10.1038/nn1172; PMID: 14703572
- de AndaFC, MeletisK, GeX, ReiD, TsaiL-H. Centrosome motility is essential for initial axon formation in the neocortex. J Neurosci2010; 30:10391 - 406; http://dx.doi.org/10.1523/JNEUROSCI.0381-10.2010; PMID: 20685982
- AndersenEF, HalloranMC. Centrosome movements in vivo correlate with specific neurite formation downstream of LIM homeodomain transcription factor activity. Development2012; 139:3590 - 9; http://dx.doi.org/10.1242/dev.081513; PMID: 22899847
- SalieR, NiederkoflerV, ArberS. Patterning molecules; multitasking in the nervous system. Neuron2005; 45:189 - 92; PMID: 15664170
- HigginbothamH, EomT-Y, MarianiLE, BachledaA, HirtJ, GukassyanV, CusackCL, LaiC, CasparyT, AntonES. Arl13b in primary cilia regulates the migration and placement of interneurons in the developing cerebral cortex. Dev Cell2012; 23:925 - 38; http://dx.doi.org/10.1016/j.devcel.2012.09.019; PMID: 23153492
- MurcianoA, ZamoraJ, López-SánchezJ, FradeJM. Interkinetic nuclear movement may provide spatial clues to the regulation of neurogenesis. Mol Cell Neurosci2002; 21:285 - 300; http://dx.doi.org/10.1006/mcne.2002.1174; PMID: 12401448
- Del BeneF, WehmanAM, LinkBA, BaierH. Regulation of neurogenesis by interkinetic nuclear migration through an apical-basal notch gradient. Cell2008; 134:1055 - 65; http://dx.doi.org/10.1016/j.cell.2008.07.017; PMID: 18805097
- HatakeyamaJ, WakamatsuY, NagafuchiA, KageyamaR, ShigemotoR, ShimamuraK. Cadherin-based adhesions in the apical endfoot are required for active Notch signaling to control neurogenesis in vertebrates. Development2014; 141:1671 - 82; http://dx.doi.org/10.1242/dev.102988; PMID: 24715457
- MizuharaE, NakataniT, MinakiY, SakamotoY, OnoY, TakaiY. MAGI1 recruits Dll1 to cadherin-based adherens junctions and stabilizes it on the cell surface. J Biol Chem2005; 280:26499 - 507; http://dx.doi.org/10.1074/jbc.M500375200; PMID: 15908431
- FoxJW, LampertiED, EkşioğluYZ, HongSE, FengY, GrahamDA, SchefferIE, DobynsWB, HirschBA, RadtkeRA, et al. Mutations in filamin 1 prevent migration of cerebral cortical neurons in human periventricular heterotopia. Neuron1998; 21:1315 - 25; http://dx.doi.org/10.1016/S0896-6273(00)80651-0; PMID: 9883725
- ItohY, MoriyamaY, HasegawaT, EndoTA, ToyodaT, GotohY. Scratch regulates neuronal migration onset via an epithelial-mesenchymal transition-like mechanism. Nat Neurosci2013; 16:416 - 25; http://dx.doi.org/10.1038/nn.3336; PMID: 23434913
- XueB, KrishnamurthyK, AllredDC, MuthuswamySK. Loss of Par3 promotes breast cancer metastasis by compromising cell-cell cohesion. Nat Cell Biol2013; 15:189 - 200; http://dx.doi.org/10.1038/ncb2663; PMID: 23263278
- HassounahNB, BunchTA, McDermottKM. Molecular pathways: the role of primary cilia in cancer progression and therapeutics with a focus on Hedgehog signaling. Clin Cancer Res2012; 18:2429 - 35; http://dx.doi.org/10.1158/1078-0432.CCR-11-0755; PMID: 22415315
- PlotnikovaOV, GolemisEA, PugachevaEN. Cell cycle-dependent ciliogenesis and cancer. Cancer Res2008; 68:2058 - 61; http://dx.doi.org/10.1158/0008-5472.CAN-07-5838; PMID: 18381407
- NietoMA. Epithelial plasticity: a common theme in embryonic and cancer cells. Science2013; 342:1234850; http://dx.doi.org/10.1126/science.1234850; PMID: 24202173
