Abstract
Adult neurogenesis occurs within a specialized microenvironment (or niche), and is dynamically regulated by experience. However, the niche mechanism that couples neuronal circuitry activity to the production of new neurons is not well-understood. In our recent two studies published in Nature1 and Nature Neuroscience,2 we identified a novel niche mechanism involving parvalbumin (PV+) interneurons that couples local circuit activity to diametric regulation of the activation of quiescent neural stem cells (NSC) and the survival of their proliferating neural progeny, two critical early phases of adult hippocampal neurogenesis. Using a combination of optogenetics, lineage-tracing of adult NSCs, and retroviral mediated approach for birthdating of neural progenitors, we demonstrated that activation of PV+ interneurons, but not somatostatin (SST+) or vasoactive intestinal peptide (VIP+) interneurons, suppresses the activation of quiescent NSCs, while simultaneously promotes the survival of proliferative newborn progeny in the adult mouse dentate gyrus. In this commentary, we review our findings and discuss a potential role for PV+ neuron-mediated GABA signaling as a key neuronal activity sensor that translates animal's experience into regulation of adult hippocampal neurogenesis.
Introduction
Neurogenesis occurs throughout life in discrete regions of the adult mammalian brain and substantial evidence supports critical roles of adult-born neurons in specific brain functions, such as learning, memory and olfactory processing (reviewed in refs. Citation3–Citation6). Under normal physiological conditions, active adult neurogenesis is limited to two brain regions, the olfactory bulb where newborn neurons arise from the subventricular zone (SVZ) of the lateral ventricles, and the dentate granule cell layer of the hippocampus where newborn neurons are generated locally within the subgranular zone (SGZ). Our current understanding of what makes these two neurogenic regions receptive to the integration of de novo neuronal populations in the adult brain is very limited. Therefore, identifying critical features of the neurogenic niche that foster synaptic integration and survival of newborn progeny may lead to novel strategies to enhance functional repair from endogenous or transplanted neurons after injury and degenerative neurological disorders.
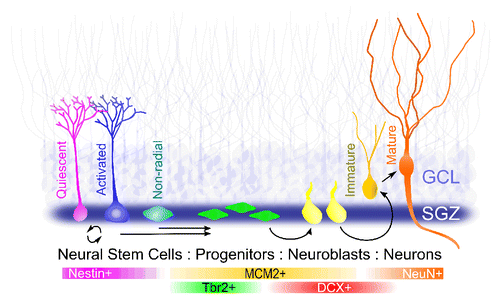
Significant progress has been made in identifying major milestones and processes underlying adult neurogenesis (reviewed in ref. Citation7). In the adult mouse dentate gyrus, using cell-stage dependent markers (nestin, Tbr2, DCX, and NeuN) and cell proliferation marker MCM2 via lineage-tracing, we showed that quiescent nestin+MCM2− NSCs give rise to highly proliferative Tbr2+MCM2+ intermediate progenitors, which in turn generate mitotic DCX+MCM2+ neuroblasts to become DCX+MCM2− immature post-mitotic neurons and finally DCX−NeuN+ mature dentate granule neurons (). Electrophysiological studies have further revealed a stereotypic process of synaptic integration of new neurons into the existing circuitry. In the adult mice, newborn neurons are tonically activated by ambient GABA soon after birth, followed by depolarizing GABAergic synaptic inputs when cells are around 1 wk old, and finally glutamatergic synaptic inputs that emerge 1 wk later.Citation8
Figure 1. Adult neurogenesis in the dentate gyrus of the hippocampus. Shown is a schematic summary of the development of newborn cells as characterized by expression of specific molecular markers at each stage.
It is generally believed that there exists a specialized local environment, or niche, that both houses somatic stem cells and regulates their development in vivo. Niche constituents that support adult SVZ or SGZ neurogenesis include endothelial cells, ependymal cells, astrocytes, microglia, and mature neurons (reviewed in refs. Citation9 and Citation10). Niche mechanisms underlying activity-dependent regulation of adult neurogenesis are not well understood. We recently showed that PV+ interneurons in the adult hippocampus, via a non-synaptic mechanism through GABA, suppress quiescent radial neural stem cell activation.Citation1 In sharp contrast to this inhibitory role of PV+ neuron activity on quiescent NSC activation, optogenetic analyses further showed that PV+ neuron activation promotes the survival of proliferating neuronal progeny.Citation2 Together, these studies identified a novel activity-dependent niche signaling mechanism through which adult neurogenesis is dynamically controlled, and revealed a diametric mode of regulation of two critical early phases of adult hippocampal neurogenesis via PV+ neuron activity. Here, we comment on how GABA signaling mediated by PV+ neurons regulates adult neurogenesis at distinct developmental stages, and discuss a potential role for PV+ neuron-mediated GABA signaling as a sensor of local neuronal circuitry activity and as a key regulator of the speed and extent of adult mammalian neurogenesis.
PV+ Interneurons as a Major Source of GABAergic Influence for Adult NSCs and Progenitors
Hippocampal microcircuits recruit different types of GABAergic interneurons, which exert inhibitory control at the soma, proximal dendrites, distal dendrites, and the axonal initial segment of the principal cells.Citation11,Citation12 The hippocampal interneurons are remarkably diverse in their location, morphology, targets, and expression of proteins, such as calcium binding proteins.Citation11,Citation13 A number of interneurons with their axons in close proximity to the SGZ neurogenic niche can potentially exert functional impact on adult neurogenesis. These interneurons include molecular layer perforant path interneurons (MOPP), hilar commissural-associational pathway related interneurons (HICAP), hilar perforant path-associated interneurons (HIPP), stratum lacunosum–moleculare (L–M) interneurons, and basket cells.Citation11,Citation12,Citation14
In contrast to embryonic neurogenesis, one hallmark of adult neurogenesis is its dynamic regulation by experience at specific stages. For example, exposure to enriched environment promotes adult hippocampal neurogenesis by increasing new neuron survival.Citation15 Using an optogenetic approach, we identified PV+ interneurons as a critical niche component that couples neuronal circuitry activity to direct regulation of early stages of adult neurogenesis in vivo under both normal physiological conditions and after experience, such as social isolation and enriched environment.Citation16 While SST+ and VIP+ interneurons also extend elaborate processes in the subgranular zone and surrounding areas, their activities did not modulate quiescent NSC behavior under identical experimental conditions, suggesting a specific role of PV+ interneurons as a niche component. PV+ interneurons are abundant in the hippocampus, representing ~20% of all GABAergic neurons, and have been implicated in higher brain function and cognitive dysfunction.Citation11,Citation17 In the adult dentate gyrus, PV+ interneurons receive excitatory inputs from dentate granule cells and, to a lesser extent, from the entorhinal cortical input.Citation18 They project axons to the dentate granule cell layer with extensive coverage across both transverse and septotemporal axes.Citation19 A common feature of PV+ interneurons is the formation of ensembles coupled by both electrical, through GAP junctions, and chemical connections through reciprocal innervations.Citation20 Among multiple subtypes of interneurons in the adult dentate gyrus, PV+ interneurons may be uniquely poised to convey relevant information about environmental stimuli and hippocampal activity to adult neurogenesis. PV+ neurons are involved in feedforward and feedback inhibition of local principal neurons in the hippocampus, they are also coupled with each other, and can fire synchronously. PV+ neurons can also indirectly regulate adult hippocampal neurogenesis via modulating its downstream targets. For example, decreased PV+ neuron activity may increase activation of mature dentate granule neurons, which in turn promotes adult hippocampal neurogenesis via release of niche factors.Citation21,Citation22
PV+ Neuron-Mediated GABA Signaling in Early Stages of Adult Neurogenesis Regulation
Tonic GABA signaling regulates quiescent NSCs
Using a combination of optogenetics to selectively target local interneuron populations and a clonal lineage-tracing system to selectively target individual quiescent NSCs (also referred as “radial glia-like cell”), we identified PV+ interneurons as a critical and unique niche component among several different interneuron subtypes that couples neuronal circuitry activity to regulate NSC quiescence and activation through γ2-containing GABAARs in the adult mouse dentate gyrus. In contrast to the direct synaptic inputs identified onto immature neurons in the adult mouse dentate gyrus,Citation23 no apparent functional GABAergic synaptic responses were detected when quiescent NSCs were recorded in acute slices in this and previous studies,Citation24 suggesting that GABA spillover from activated PV+ interneuron-mature granule cell synapses indirectly regulates nearby NSCs. The tonic signal has two very intriguing attributes that make its involvement in neurogenesis regulation especially attractive. First, because tonic signaling transcends individual pre-synaptic/post-synaptic cellular pairs, it provides a means for acting on cells that might be located some distance from the proximity of the signaling synapse. Second, recurrent connections are extensive between granule cells and interneurons in the SGZ microenvironment and since the ambient transmitter level is an integrated signal, it is an especially attractive candidate signal that reflects the overall local network activity for potential translation by the neural progenitors. The neurogenic SGZ region is in a mesh of interneuron terminals; therefore, adult NSCs are subject to dynamic regulation of various interneuron types possibly through neurotransmitter GABA. Interestingly, a recent study showed that tonic and phasic GABA activation of neural progenitor cells and immature neurons is modulated by chemokine stromal cell-derived factor 1 co-released with GABA from local interneurons.Citation25 While the mechanisms underlying such a regulation remain to be determined, these findings suggest that additional specificity of regulation could be achieved by co-factors in the local niche.
Synaptic GABA signaling regulates proliferating progenitor survival
Significant progress has been made in characterizing synaptic integration of newborn neurons into the existing neuronal circuitry (reviewed in ref. Citation26), yet little is known about the properties of the first functional synaptic inputs formed onto newborn progeny during adult hippocampal neurogenesis. Previous electron microscopy and electrophysiological analyses have suggested the presence of morphological synapses onto early precursors in the postnatal SGZ.Citation24,Citation27,Citation28 In agreement with these early observations, we further identified one source of presynaptic inputs from PV+ neurons, and these inputs are acting on the proliferating progenitors. This result is surprising because previous studies have suggested that PV+ neurons make functional synaptic contacts with neurons largely upon maturation of target neurons.Citation29 In contrast to highly efficient synapses from PV+ interneurons onto mature dentate granule neurons where GABA release can be induced with activation of three or fewer Ca2+ channels,Citation30 these initial PV+ synapses onto newborn progeny exhibit variable structural forms and appear to be functionally immature and are only activated upon a train of stimuli.
What is the functional role of these immature synapses in regulation of neural progenitors at the proliferating phase? Computational models have suggested advantages of circuit activity-neurogenesis coupling for temporal storage and memory clearing in the adult hippocampusCitation31,Citation32 and for tuning olfactory bulb activity to improve odorant discrimination.Citation33 Recent experimental evidence also suggests that a proper rate of both addition and elimination of new neurons optimizes behavioral outcomes in animal models.Citation34-Citation36 At the cellular level, previous studies of adult hippocampal neurogenesis have identified an activity-dependent phase of competitive survival of 2- to 3-wk-old post-mitotic new neurons via glutamate-NMDAR signaling.Citation37 This critical phase of neuronal elimination occurs during glutamatergic synapse formation and is input-specific. In addition to this activity-dependent fine-tuning of post-mitotic new neuron number, we show a large-scale regulation of neuronal production at the proliferative phase that is also circuitry activity-dependent, but via GABA signaling. Notably, this activity-dependent mechanism appears to be distinct from that during embryonic neurogenesis when cell death of proliferating neural progenitors occurs before any synapse formation. During adult SVZ neurogenesis, significant cell death has also been observed in the rostral migratory stream,Citation38,Citation39 although the role of neuronal circuitry activity in this process is unknown. In addition, a second phase of new neuron number regulation occurs during experience-regulated synaptic integration into olfactory bulb circuitry.Citation40 Thus, multi-phasic activity-dependent control of new neuron numbers may be a general rule for adult neurogenesis, starting with robust regulation during the proliferative phase and followed by refinement of individual neuron survival during the maturation phase.
Local Circuitry Control of Adult Hippocampal Neurogenesis—Diametric Regulation of Adult Neurogenesis by Local PV+ Interneurons
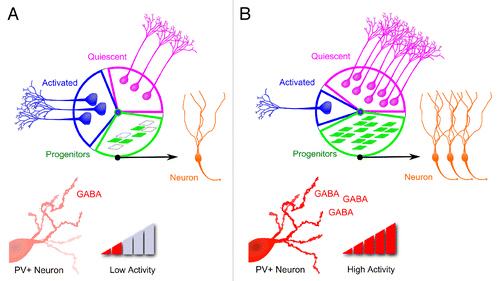
PV+ neuron activity that promotes proliferating neural progeny survival is in sharp contrast to its inhibitory role on quiescent neural stem cell activation in adult dentate gyrus.Citation1 Together, these results reveal a diametric mode of adult hippocampal neurogenesis regulation via PV+ neuron activity (): during heightened activity within dentate gyrus, activation of PV+ neurons promotes the survival of proliferating neuronal progenitors and inhibits quiescent neural stem cell activation; conversely, when the activity in the dentate gyrus is low, decreased PV+ neuron activity suppresses the survival of proliferating neuronal progenitors and simultaneously promotes the activation of quiescent NSCs via symmetric cell division. Consequently, sustained low PV+ neuron activity is expected to expand the quiescent NSC pool at the expenses of decreased neurogenesis presumably by decreasing proliferating progenitor survival. In contrast, sustained high PV+ neuron activity is expected to increase neurogenesis by promoting proliferating progenitor survival and preventing quiescent NSCs from activation thereby preserving the quiescent stem cell pool for long-term sustainable neurogenesis.
Figure 2. Diametric regulation of two sequential proliferative processes of adult hippocampal neurogenesis by PV+ neuron activity. Shown is a model of diametric regulation of quiescent neural stem cell activation, and survival and maturation of their proliferative neuronal progeny: during low activity within dentate gyrus (A), decreased PV+ neuron activity suppresses the survival of proliferating neuronal progenitors and simultaneously promotes expansion of the neural stem cell pool via symmetric cell division; conversely, when the activity in the dentate gyrus is high (B), activation of PV+ neurons promotes the survival and maturation of proliferating neuronal progenitors and inhibits quiescent neural stem cell activation.
The potential advantage of such a diametric mode of neuronal circuitry–neurogenesis coupling may be associated with adaptive adult neurogenesis regulation in response to dynamic network activity. Low levels of neuronal activity in the dentate gyrus may make it unnecessary for additional newborn neurons to integrate into the existing circuitry. Meanwhile, expanding the NSC pool via symmetric cell division during periods of low PV+ neuron activity may prepare the dentate gyrus for future adaptive neurogenesis where the activity returns and NSCs may undergo asymmetric cell division to produce more new neurons. Conversely, heightened dentate gyrus activity leads to a decrease of quiescent NSC activation. Such decreased activation of NSCs can potentially prevent stem cell depletion to ensure lifelong neurogenesis.Citation41,Citation42
In addition, it may facilitate time-stamping of a specific cohort of newborn neurons during heightened activity of dentate gyrus by increasing the cohort of recently born neuronal progeny while simultaneously suppressing the generation of new neurons of a different age from quiescent NSC activation. This could be particularly important, given recent behavioral studies suggesting a critical contribution of newborn neurons at a particular developmental stage to hippocampal function.Citation43-Citation45
Experience-Dependent Regulation of Adult Neurogenesis through Local Interneurons
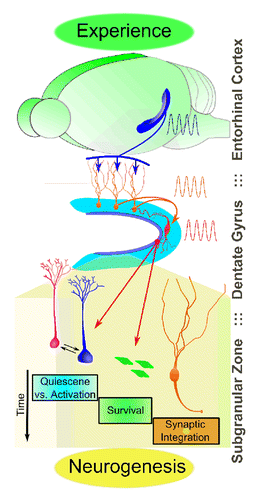
Different from other somatic stem cell compartments where morphogens and growth factors generally serve as niche signals, adult neurogenesis is well known to be dynamically regulated by neuronal activity and experience. Accumulating evidence suggests that different steps of adult neurogenesis are differentially regulated by physiological and pathological stimuli, including an enriched environment, stress, learning, and seizures (reviewed in ref. Citation9). Although these studies indicate that neuronal activity is essential for this adult form of structural plasticity, how electrical activity directly regulates neural stem cells and their progeny is largely unknown. As adult hippocampal neurogenesis occurs within a dynamic neuronal network, it is conceivable that the local circuit activity could serve as an effective readout of current tissue demands and provide a signal to tune new neuron production. Indeed, our results suggest a model that PV+ neurons serve as a unique niche component to couple environmental stimuli to the regulation of adult NSCs and neural progenitors. Specifically, decreased PV+ neuron activity by chronic social isolation promotes excessive activation and symmetric cell division of NSCs at the expenses of decreased neuronal production,Citation1 whereas increased PV+ neuron activity by exposure to an enriched environment promotes the survival of both proliferating neural progenitors and subsequent mature neurons.Citation2 Though the causal link between altered neurogenesis and animal behavior has not been established, these findings point to the possibility that neuronal activity modulation by local circuits may provide the fine control of synaptic integration of adult-born neurons required for specific neurogenic events in response to experiences, environmental influence, and pathological conditions ().
Figure 3. Experience-dependent regulation of adult neurogenesis mediated by local interneurons. Shown is an illustration of how local interneurons may couple experiential activity to the regulation of adult neurogenesis at distinct developmental stages. Entorhinal cortical inputs activate dentate granule neurons, which in turn activate local PV+ interneurons. Local PV+ interneurons serve as intermediary cells which relay signals to adult neural stem cells and their progeny.
Summary
Our studies identified a critical role of PV+ neurons as a key sensor of local neuronal circuitry activity to regulate early phases of adult hippocampal neurogenesis in response to experience. Given the extensive evidence of regulations in both the number and properties of PV+ interneurons in the adult hippocampus under many physiological and pathological conditions, such as aging, Alzheimer diseases, chronic stress, schizophrenia and other severe psychiatric illness, and epilepsy,Citation46 these findings have significant and broad implications. In addition, a lack of such niche mechanisms in “non-neurogenic” regions may impede functional integration and survival of new neurons that have migrated to injury sites. Mimicking basic features of this niche mechanism may constitute a novel strategy to overcome deficits in early integration and neuronal survival of either endogenous or transplanted cells to promote adult plasticity and regeneration in brain disorders and after injury.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The research in the authors’ laboratories was supported by UNC start-up fund (J.S.), NARSAD (J.S. and G.M.), Whitehall Foundation (J.S.), American Heart Association (J.S.), UNC BBSP and Neurobiology Curriculum training grant (A.J.C.), UNC Department of Pharmacology training grant (R.H.J.O.), NIH (G.M. and H.S.), and MSCRF (G.M.).
References
- SongJ, ZhongC, BonaguidiMA, SunGJ, HsuD, GuY, MeletisK, HuangZJ, GeS, EnikolopovG, et al. Neuronal circuitry mechanism regulating adult quiescent neural stem-cell fate decision. Nature2012; 489:150 - 4; http://dx.doi.org/10.1038/nature11306; PMID: 22842902
- SongJ, SunJ, MossJ, WenZ, SunGJ, HsuD, ZhongC, DavoudiH, ChristianKM, ToniN, et al. Parvalbumin interneurons mediate neuronal circuitry-neurogenesis coupling in the adult hippocampus. Nat Neurosci2013; 16:1728 - 30; http://dx.doi.org/10.1038/nn.3572; PMID: 24212671
- ZhaoC, DengW, GageFH. Mechanisms and functional implications of adult neurogenesis. Cell2008; 132:645 - 60; http://dx.doi.org/10.1016/j.cell.2008.01.033; PMID: 18295581
- LledoPM, AlonsoM, GrubbMS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci2006; 7:179 - 93; http://dx.doi.org/10.1038/nrn1867; PMID: 16495940
- SongJ, ChristianKM, MingGL, SongH. Modification of hippocampal circuitry by adult neurogenesis. Dev Neurobiol2012; 72:1032 - 43; http://dx.doi.org/10.1002/dneu.22014; PMID: 22354697
- BraunSG, JessbergerS. Adult neurogenesis in the mammalian brain. Front Biol2013; 8:295 - 304; http://dx.doi.org/10.1007/s11515-013-1263-1
- MingGL, SongH. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron2011; 70:687 - 702; http://dx.doi.org/10.1016/j.neuron.2011.05.001; PMID: 21609825
- GeS, GohEL, SailorKA, KitabatakeY, MingGL, SongH. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature2006; 439:589 - 93; http://dx.doi.org/10.1038/nature04404; PMID: 16341203
- MaDK, KimWR, MingGL, SongH. Activity-dependent extrinsic regulation of adult olfactory bulb and hippocampal neurogenesis. Ann N Y Acad Sci2009; 1170:664 - 73; http://dx.doi.org/10.1111/j.1749-6632.2009.04373.x; PMID: 19686209
- MaDK, MingGL, SongH. Glial influences on neural stem cell development: cellular niches for adult neurogenesis. Curr Opin Neurobiol2005; 15:514 - 20; http://dx.doi.org/10.1016/j.conb.2005.08.003; PMID: 16144763
- FreundTF, BuzsákiG. Interneurons of the hippocampus. Hippocampus1996; 6:347 - 470; http://dx.doi.org/10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I; PMID: 8915675
- HouserCR. Interneurons of the dentate gyrus: an overview of cell types, terminal fields and neurochemical identity. Prog Brain Res2007; 163:217 - 32; http://dx.doi.org/10.1016/S0079-6123(07)63013-1; PMID: 17765721
- MaccaferriG, LacailleJC. Interneuron Diversity series: Hippocampal interneuron classifications--making things as simple as possible, not simpler. Trends Neurosci2003; 26:564 - 71; http://dx.doi.org/10.1016/j.tins.2003.08.002; PMID: 14522150
- HanZS, BuhlEH, LörincziZ, SomogyiP. A high degree of spatial selectivity in the axonal and dendritic domains of physiologically identified local-circuit neurons in the dentate gyrus of the rat hippocampus. Eur J Neurosci1993; 5:395 - 410; http://dx.doi.org/10.1111/j.1460-9568.1993.tb00507.x; PMID: 8261117
- KempermannG, KuhnHG, GageFH. More hippocampal neurons in adult mice living in an enriched environment. Nature1997; 386:493 - 5; http://dx.doi.org/10.1038/386493a0; PMID: 9087407
- DranovskyA, PicchiniAM, MoadelT, SistiAC, YamadaA, KimuraS, LeonardoED, HenR. Experience dictates stem cell fate in the adult hippocampus. Neuron2011; 70:908 - 23; http://dx.doi.org/10.1016/j.neuron.2011.05.022; PMID: 21658584
- BartosM, VidaI, JonasP. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci2007; 8:45 - 56; http://dx.doi.org/10.1038/nrn2044; PMID: 17180162
- SeressL, AbrahámH, PaleszterM, GallyasF. Granule cells are the main source of excitatory input to a subpopulation of GABAergic hippocampal neurons as revealed by electron microscopic double staining for zinc histochemistry and parvalbumin immunocytochemistry. Exp Brain Res2001; 136:456 - 62; http://dx.doi.org/10.1007/s002210000601; PMID: 11291726
- StrubleRG, DesmondNL, LevyWB. Anatomical evidence for interlamellar inhibition in the fascia dentata. Brain Res1978; 152:580 - 5; http://dx.doi.org/10.1016/0006-8993(78)91113-7; PMID: 687975
- FreundTF. Interneuron Diversity series: Rhythm and mood in perisomatic inhibition. Trends Neurosci2003; 26:489 - 95; http://dx.doi.org/10.1016/S0166-2236(03)00227-3; PMID: 12948660
- FavaroR, ValottaM, FerriAL, LatorreE, MarianiJ, GiachinoC, LanciniC, TosettiV, OttolenghiS, TaylorV, et al. Hippocampal development and neural stem cell maintenance require Sox2-dependent regulation of Shh. Nat Neurosci2009; 12:1248 - 56; http://dx.doi.org/10.1038/nn.2397; PMID: 19734891
- StoneSS, TeixeiraCM, DevitoLM, ZaslavskyK, JosselynSA, LozanoAM, FranklandPW. Stimulation of entorhinal cortex promotes adult neurogenesis and facilitates spatial memory. J Neurosci2011; 31:13469 - 84; http://dx.doi.org/10.1523/JNEUROSCI.3100-11.2011; PMID: 21940440
- MarkwardtSJ, WadicheJI, Overstreet-WadicheLS. Input-specific GABAergic signaling to newborn neurons in adult dentate gyrus. J Neurosci2009; 29:15063 - 72; http://dx.doi.org/10.1523/JNEUROSCI.2727-09.2009; PMID: 19955357
- WangLP, KempermannG, KettenmannH. A subpopulation of precursor cells in the mouse dentate gyrus receives synaptic GABAergic input. Mol Cell Neurosci2005; 29:181 - 9; http://dx.doi.org/10.1016/j.mcn.2005.02.002; PMID: 15911343
- BhattacharyyaBJ, BanisadrG, JungH, RenD, CronshawDG, ZouY, MillerRJ. The chemokine stromal cell-derived factor-1 regulates GABAergic inputs to neural progenitors in the postnatal dentate gyrus. J Neurosci2008; 28:6720 - 30; http://dx.doi.org/10.1523/JNEUROSCI.1677-08.2008; PMID: 18579746
- GeS, SailorKA, MingGL, SongH. Synaptic integration and plasticity of new neurons in the adult hippocampus. J Physiol2008; 586:3759 - 65; http://dx.doi.org/10.1113/jphysiol.2008.155655; PMID: 18499723
- TozukaY, FukudaS, NambaT, SekiT, HisatsuneT. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron2005; 47:803 - 15; http://dx.doi.org/10.1016/j.neuron.2005.08.023; PMID: 16157276
- KaplanMS, BellDH. Mitotic neuroblasts in the 9-day-old and 11-month-old rodent hippocampus. J Neurosci1984; 4:1429 - 41; PMID: 6726341
- EspósitoMS, PiattiVC, LaplagneDA, MorgensternNA, FerrariCC, PitossiFJ, SchinderAF. Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J Neurosci2005; 25:10074 - 86; http://dx.doi.org/10.1523/JNEUROSCI.3114-05.2005; PMID: 16267214
- BucurenciuI, BischofbergerJ, JonasP. A small number of open Ca2+ channels trigger transmitter release at a central GABAergic synapse. Nat Neurosci2010; 13:19 - 21; http://dx.doi.org/10.1038/nn.2461; PMID: 20010820
- DeisserothK, SinglaS, TodaH, MonjeM, PalmerTD, MalenkaRC. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron2004; 42:535 - 52; http://dx.doi.org/10.1016/S0896-6273(04)00266-1; PMID: 15157417
- ChambersRA, PotenzaMN, HoffmanRE, MirankerW. Simulated apoptosis/neurogenesis regulates learning and memory capabilities of adaptive neural networks. Neuropsychopharmacology2004; 29:747 - 58; http://dx.doi.org/10.1038/sj.npp.1300358; PMID: 14702022
- CecchiGA, PetreanuLT, Alvarez-BuyllaA, MagnascoMO. Unsupervised learning and adaptation in a model of adult neurogenesis. J Comput Neurosci2001; 11:175 - 82; http://dx.doi.org/10.1023/A:1012849801892; PMID: 11717533
- SahayA, ScobieKN, HillAS, O’CarrollCM, KheirbekMA, BurghardtNS, FentonAA, DranovskyA, HenR. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature2011; 472:466 - 70; http://dx.doi.org/10.1038/nature09817; PMID: 21460835
- KimWR, ParkOH, ChoiS, ChoiSY, ParkSK, LeeKJ, RhyuIJ, KimH, LeeYK, KimHT, et al. The maintenance of specific aspects of neuronal function and behavior is dependent on programmed cell death of adult-generated neurons in the dentate gyrus. Eur J Neurosci2009; 29:1408 - 21; http://dx.doi.org/10.1111/j.1460-9568.2009.06693.x; PMID: 19519627
- MouretA, LepousezG, GrasJ, GabellecMM, LledoPM. Turnover of newborn olfactory bulb neurons optimizes olfaction. J Neurosci2009; 29:12302 - 14; http://dx.doi.org/10.1523/JNEUROSCI.3383-09.2009; PMID: 19793989
- TashiroA, SandlerVM, ToniN, ZhaoC, GageFH. NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature2006; 442:929 - 33; http://dx.doi.org/10.1038/nature05028; PMID: 16906136
- MorsheadCM, van der KooyD. Postmitotic death is the fate of constitutively proliferating cells in the subependymal layer of the adult mouse brain. J Neurosci1992; 12:249 - 56; PMID: 1729437
- PlatelJC, DaveKA, GordonV, LacarB, RubioME, BordeyA. NMDA receptors activated by subventricular zone astrocytic glutamate are critical for neuroblast survival prior to entering a synaptic network. Neuron2010; 65:859 - 72; http://dx.doi.org/10.1016/j.neuron.2010.03.009; PMID: 20346761
- LepousezG, LledoPM. Life and death decision in adult neurogenesis: in praise of napping. Neuron2011; 71:768 - 71; http://dx.doi.org/10.1016/j.neuron.2011.08.014; PMID: 21903071
- BonaguidiMA, WheelerMA, ShapiroJS, StadelRP, SunGJ, MingGL, SongH. In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell2011; 145:1142 - 55; http://dx.doi.org/10.1016/j.cell.2011.05.024; PMID: 21664664
- EncinasJM, MichurinaTV, PeunovaN, ParkJH, TordoJ, PetersonDA, FishellG, KoulakovA, EnikolopovG. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell2011; 8:566 - 79; http://dx.doi.org/10.1016/j.stem.2011.03.010; PMID: 21549330
- AimoneJB, DengW, GageFH. Resolving new memories: a critical look at the dentate gyrus, adult neurogenesis, and pattern separation. Neuron2011; 70:589 - 96; http://dx.doi.org/10.1016/j.neuron.2011.05.010; PMID: 21609818
- SahayA, WilsonDA, HenR. Pattern separation: a common function for new neurons in hippocampus and olfactory bulb. Neuron2011; 70:582 - 8; http://dx.doi.org/10.1016/j.neuron.2011.05.012; PMID: 21609817
- GuY, Arruda-CarvalhoM, WangJ, JanoschkaSR, JosselynSA, FranklandPW, GeS. Optical controlling reveals time-dependent roles for adult-born dentate granule cells. Nat Neurosci2012; 15:1700 - 6; http://dx.doi.org/10.1038/nn.3260; PMID: 23143513
- MasiulisI, YunS, EischAJ. The interesting interplay between interneurons and adult hippocampal neurogenesis. Mol Neurobiol2011; 44:287 - 302; http://dx.doi.org/10.1007/s12035-011-8207-z; PMID: 21956642
