Abstract
The extensive and multifaceted traffic between nucleus and cytoplasm is handled by a single type of macromolecular assembly called the nuclear pore complex (NPC). While being readily accessible to ions and metabolites, the NPC imposes stringent selectivity on the passage of proteins and RNA, tightly regulating their traffic between the two major cellular compartments. Here we discuss how shuttling carriers, which mediate the transport of macromolecules through NPCs, cross its permeability barrier. We also discuss the co-existence of receptor-mediated macromolecular transport with the passive diffusion of small molecules in the context of the various models suggested for the permeability barrier of the NPC. Finally, we speculate on how nuclear transport receptors negotiate the dependence of their NPC-permeating abilities on hydrophobic interactions with the necessity of avoiding these promiscuous interactions in the cytoplasm and nucleus.
The division of eukaryotic cells into multiple compartments makes separation of cellular functions possible by devoting membrane-enclosed volumes specific to them. While this provides many advantages, it also presents a challenge, as some means of communication between each compartment and the rest of the cell must be made available without compromising its integrity. In most cases, a multitude of channels, transporters and translocons, each dedicated to trafficking specific molecules, is embedded within the membranes that enclose cellular compartments. But the heaviest traffic of all, the one between the nucleus and the cytoplasm in interphase cells, proceeds through a single type of conduit—the nuclear pore complex (NPC), a mammoth proteinaceous assembly that spans the nuclear envelope and provides the sole means for the exchange of material and information between the two compartments (). This exclusivity poses a functional dilemma: while some molecules, such as ions and metabolites, need to be constantly shuttled between the two compartments, the traffic of others, namely of proteins and RNAs, must be tightly controlled as the unscheduled nuclear entry or exit of many of them could be highly detrimental. Thus, each NPC must be continuously conductive to certain molecules and at the same time impermeable to others. Given that some of the cargoes that cross the NPC can be quite substantial, e.g., mRNAs or ribosomal subunits, achieving the task of being open and closed simultaneously requires some engineering ingenuity.
Part of this dual requirement problem is resolved by a size-exclusion mechanism. Molecules smaller than ∼5 nm in diameter can cross NPCs unassisted, by passive diffusion, which becomes increasingly restricted as the size of the molecule approaches the cut-off limit of the filtration device.Citation1,Citation2 This permits water, ions and metabolites to enter and exit the nucleus freely, while preventing transport of larger molecules. The passage of proteins and RNAs, whose size is typically above the cut-off limit, is achieved by association with mobile carriers, which recognize those molecules that are destined for transport and selectively pass them through the NPC, this without compromising the selectivity of the pore towards other molecules. The shuttling carriers that have been selected for this task are primarily superhelical, flexible proteins belonging to the karyopherin β/importin β superfamily,Citation3–Citation5 which are uniquely decorated by hydrophobic patches on their surface.Citation6–Citation12 These mediators, referred to here as nuclear transport receptors (NTRs), appear to be tailor made for the job, as they have no obvious homologues in prokaryotes.Citation13 NTRs recognize their cargoes, directly or with the aid of adapter proteins, through the presence of semi-conserved amino acid sequences coined NLS (nuclear localisation signals) or NES (nuclear export signals), which mark the cargo for nuclear import or export (or both).Citation14–Citation17 Given the number of different proteins (including those that piggy-back RNA molecules) that must enter or exit the cell nucleus, this generic tagging scheme avoids unrealistic multiplicity of cargo-specific NTRs.
Once a cargo has reached its destination compartment it must dissociate from the NTR. The assembly and disassembly of cargo-receptor complexes is orchestrated by the small Ras-like GTPase Ran, that, when loaded with GTP, promotes dissociation of import complexes and association of export complexes (). Segregation of Ran's nucleotide exchange factor (GEF) to the nucleus, and GTPase activating protein (GAP) to the cytoplasm, generates a concentration gradient of RanGTP across the nuclear membrane. The latter, together with the opposing effects of RanGTP on the affinity of import and export transport receptors towards their cargoes, imposes overall directionality to transport by ensuring that cargo is loaded and released in the appropriate cellular compartment. Maintaining the RanGTP gradient requires the input of metabolic energy, in the form of hydrolysis of Ran-bound GTP in cytoplasm, and is aided by two Ran-binding proteins, called RanBP1 and RanBP2, which facilitate the RanGAP-stimulated hydrolysis of the Ran-bound GTP. The process also requires the Ran-dedicated nuclear import receptor, NTF2, which shuttles RanGDP to the nucleus where recharging with GTP takes place.Citation14–Citation19
What is it that allows NPCs to readily transport small molecules, exclude large molecules, and yet let the latter pass through when bound to NTRs? A large body of evidence suggests that the employed scheme relies on tandem hydrophobic sequence repeats, most commonly in the form of FG, GLFG or FXFG, separated by (more) hydrophilic spacer sequences. These repeats, which are present in natively disordered domains of many proteins that make up the NPC (called nucleoporins or Nups), specifically interact with NTRs (reviewed in ref. Citation20). The proteins carrying these so called FG-repeat domains are anchored on one side to the NPC scaffold whereas their FG-repeat-containing regions are believed to protrude into the central pore channel, cytoplasm and nucleus.Citation21 A debate still exists as to the exact form these FG-repeats take within the central channel and at the periphery of the pores, and how this form leads to selectivity. One model () asserts that FG-repeats in the central pore channel interact with each other through their hydrophobic moieties, filling it with a meshwork that has openings of about 10 nm, which enable small molecules to pass through but preclude the transport of larger inert cargoes.Citation22,Citation23 Having an enhanced surface hydrophobicity conferred by their hydrophobic patches, NTRs are able to ‘solubilise’ into the meshwork by binding to hydrophobic residues in the FG-repeats, transiently replacing contacts between them. As soon as the NTR moves further along the channel, the FG-repeats resume their contacts, sealing the mesh behind the transporting cargo. Consistent with this, saturated FG-repeat hydrogels preferentially support the passage of NTRs over comparably-sized proteins that cannot interact with FG-repeats.Citation24–Citation26 Another model submits that FG-repeat bristles that emanate from the pore towards the nucleoplasm and cytoplasm, exclude cargoes from entering the central channel by virtue of their thermal motionCitation27 (). Able to bind FG-repeats, NTR's are present in an increased concentration near the pore entrances and therefore have a greater propensity for entering it. All sorting activity is conducted outside the central channel and the authors have shown that attaching FG-repeat-containing nucleoporins (FG-Nups) to synthetic pores at their entrance allows for faster transport of NTRs compared to an inert protein of a similar size (BSA).Citation28 Using AFM and immuno-EM, Lim et al. showed that the FG-containing nucleoporin Nup153 collapses in the presence of the prototypic nuclear import receptor importin β1,Citation29,Citation30 (). The addition of RanGTP (which dissociates the receptor from FG-repeats), led to re-extension of the nucleoporin. Thus they concluded that collapse of FG-Nups in the presence of NTRs draws the cargo complex into the channel, and subsequent collapse and distension events afford its transport. Another model, coined the reduced dimensionality model, suggests that FG-repeats form a coating layer over the surface of the entire conduitCitation31 (). NTR's glide along this layer, with their effective velocity enhanced due to the fact that they are confined to a surface, and thus perform a 2D rather than 3D random walk. Inert cargo passes through a ∼10 nm-wide aqueous diffusion tube located at the middle of the central pore channel. Support for this picture can be found in a recent single-molecule study that mapped the positions of importin β-cargo complexes within the NPC, and found that they are excluded from a 20 nm-wide channel in the middle of the NPC.Citation32 Recently a model that incorporates aspects of different schemes has been proposed.Citation33 According to this model some FG-Nups tend to form extended structures whereas others tend to collapse, dividing the NPC into regions in which the FG-network takes on different forms.
The idea that the NPC contains regions varying in the form taken by the FG-Nups also fits well with its architecture. At the cytoplasmic and nuclear faces, the openings of the central channel are about 60–70 nm wide (in metazoans) with the channel narrowing at the mid-plane, thus forming an hourglass shape ().Citation34–Citation37 Further on into the nucleus, the nuclear basket is made by eight filaments which come increasingly closer together, finally binding to an approximately 40 nm wide ring.Citation37–Citation39 At the cytoplasmic side, eight filaments are radially distributed on top of the NPC body and protrude into the cytoplasm. The diameter of the conduit, and consequently, the degree of confinement of the polymers grafted to its surface thus depend on the position along the NPC central axis. In addition, the distribution of FG-repeats along the NPC is not entirely symmetricCitation21,Citation40 and the affinity of different repeats to NTR's varies substantially.Citation41–Citation45 It is feasible that the non-uniform geometry and affinity lead to a gradient in interaction strength, which can in principle, translate into local forces. A recent single-molecule studyCitation46 has demonstrated the co-existence of two populations of importin β within the NPC: One consisting of 7 molecules per NPC characterised by a dissociation constant of 0.3 nM and one involving 110 molecules per NPC, with a dissociation constant of 70 nM. These two populations could represent receptor molecules bound to different areas within the NPC that have different binding affinities to NTRs.
Regardless of the form taken by FG-repeats, it is quite clear that the selective permeation of the NPC to NTRs heavily relies on hydrophobic interactions between the latter and FG-repeats over and/or within the pores. What was not clear is whether these hydrophobic interactions are sufficient to entail access to the NPC or if additional interactions are required, whether hydrophobicity must take on a particular threshold or additive value, and whether the hydrophobic moieties recognized by the FG-repeats must be distributed in a specific manner over the NTRs surface. To tackle these questions, we constructed synthetic NTR mimics whose surface hydrophobicity had been systematically modified.Citation47 The basis for the mimics was BSA, a globular protein that is normally barred from entry to the NPC. This protein has the advantage of being cross-linked by 17 disulfide bonds, which render it extremely stable, enabling extensive modifications to be made (and, indeed, were made) without compromising its fold. We modified its surface hydrophobicity by covalently attaching three different hydrophobic amino acid side-chain analogues (Phe, Trp, Leu) to distal functional groups of solvent-exposed residues, primarily lysines (Lys) and serines (Ser). The three analogues differ both in their structure and in their chemical nature, being aromatic (Phe), polar-aromatic (Trp), and non-aromatic (Leu). The modification process was carried out at several stochiometries such that it produced different populations of mimics, each containing BSA molecules with a characteristic number of hydrophobic moieties on their surface. As controls we used unmodified BSA and BSA that was modified by a hydrophilic (Ser) side-chain analogue. Since these modifications did not significantly alter the size or the structure of the BSA, following the ability of the different protein derivatives (which were fluorescently labelled) to enter the nucleus put us in a position to single out the effect of surface hydrophobicity on the capacity of macromolecules to traverse NPC's. In addition, it allowed us to gain insight into the size and architecture of the interaction network between FG-repeats and transporting cargoes.
We first checked whether surface hydrophobicity per se is sufficient to allow a cargo to cross the NPC or whether a more specific recognition process is required. Accordingly, we studied the ability of eight different BSA derivates, containing between 4–66 hydrophobic moieties on their surface, to enter the nucleus of digitonin-permeabilised HeLa cells. We found that all of them were able to enter the nuclei regardless of the identity or number of the hydrophobic conjugates they carried. In contrast, naked BSA or BSA modified with the non-hydrophobic moiety could not pass through the NPC and were excluded from the nucleus. Thus, surface hydrophobicity in itself, and not a specifically configured interaction, suffices to compromise the permeability barrier of the NPC. The apparent promiscuity of the interactions is consistent with observations showing that FG-repeats can interact with different residues on NTRsCitation6–Citation12 and that substitutions of hydrophobic residues in FG-repeats by other hydrophobic residues does not affect the interactions between FG-repeats and NTRs.Citation45,Citation48
But is there an optimum arrangement in terms of numbers or distribution that would lead to the fastest kinetics? Assuming that all the interactions are of similar strength, one would expect that too few moieties would diminish the mimic's ability to interact with and, therefore, partition into the pore, whereas too many would slow down or even block its movement across the pore. This predicts that an optimal number of interactions should exist, such that a high occupation probability is maintained without increasing the residence time (which scales roughly exponentially with overall interaction strengthCitation49) too drastically. To test this, we followed the nuclear entry kinetics of all of the BSA derivates. Within the available range of attached conjugates, we found the kinetics to be essentially independent of the number of hydrophobic side-chains. Moreover, the t1/2 values were comparable to those measured for native NTRs. Because the cargo with the least number of conjugates contained only four hydrophobic moieties, it appears that if an optimal number of moieties indeed exists, it should lie within the range of 0–4. The above results also imply that, regardless of the number of hydrophobic patches on their surface, the number of contacts made between our mimics and the FG-repeat network is highly limited. The limit could be set, for example, by a distribution of FG-repeats that does not allow more than four simultaneous contacts with cargo to be made, or potentially by crowding in the channel which restricts the number of FG-repeat sequences available per cargo. Such scenarios have recently been corroborated in a study on an FG-repeat thin filmCitation50 and are in line with all models suggested for the FG-repeat network, provided that a limit is set on the number of concurrent interactions with cargo. Another possible explanation for the insensitivity of accumulation rate to surface hydrophobicity brings us back to the consideration placed at the beginning of this paragraph: An increased interaction strength would predictably lead to a progressively higher entry rate of cargo into the channel. At the same time, however, it would unavoidably slow down motion within the channel and release at its edge. If these opposing effects are of similar magnitudes, as for example entropy and enthalpy are in protein folding reactions, the net result would be an apparent insensitivity to the number of moieties present on the surface. One final possibility is that while selectivity depends on surface hydrophobicity, the rate-limiting step of the transport process itself does not. We have at this point no means of distinguishing between these possibilities.
Results obtained by usCitation51 and othersCitation52–Citation57 have suggested that the transport of small, passively diffusing molecules and of NTR-cargo complexes is largely segregated. This separation may be afforded by peripheral channels that were proposed to surround the central pore channel and serve as conduits for passive diffusion.Citation34,Citation39,Citation58 Another possibility is that suggested in the reduced dimensionality model (), in which receptor-mediated transport occurs along the walls of the central pore channel whereas passively diffusing cargoes make use of the aqueous diffusion tube located at the pore centre.Citation31 A conceptually similar model suggests functional division based on two zones, with one being more charged than the other.Citation33 This latter zone could accommodate passive cargoes when the central, more hydrophobic zone is heavily occupied. Interestingly, when we modified 10 kDa dextran molecules, which are able to transport passively through NPCs, with hydrophobic moieties (<10 Trp), in a manner similar to the one used for BSA, we found that they exhibited faster nuclear entry kinetics than the unmodified molecules (Naim et al. unpublished results). The kinetics was also significantly faster than that of the BSA derivates. Thus, the addition of hydrophobic conjugates allows dextran molecules to speed up, indicating either that they can now diffuse through an effectively wider channel, because they can make use of a volume of the channel that is normally not accessible to them, or that interactions with FG-repeats in the NPC facilitate their partitioning into and/or movement through the pores. Extending these observations, we propose that surface hydrophobicity, in addition to being essential for the ability of NTRs to cross the permeability barrier of the NPC, also allows the NPC to sort incoming transport substrates to different translocation pathways, dividing the loads of passively diffusing and receptor-bound species.
If the mere addition of a few hydrophobic moieties to a fairly sizable protein such as BSA allows it to efficiently traverse the NPC, why bother with specific, carefully designed, positioning of hydrophobic residues in the structure of NTRs? Part of the answer may lie in the exposure of the hydrophobic side chains to the surrounding. In NTRs, the FG-binding sites are usually located within crevices or depressionsCitation6–Citation12 that, while being relatively shallow, limit their accessibility. Close contacts between these sites and hydrophobic residues present on other proteins are therefore restricted to a limited set of local chain conformations, like those adopted by Nups' FG-repeats. This ensures that spurious interactions of NTRs with inert proteins that possess hydrophobic moieties on their surface are not formed. Such inadvertent interactions may interfere with the diffusion of NTRs in the highly crowded cytoplasmic or nucleoplasmic milieu. Indeed, the BSA derivates we used, in which the hydrophobic conjugates were attached to highly exposed distal side-chain groups, often bound to cytoplasmic components, likely cytoskeletal elements. This binding severely interfered with the movement of the mimics in the cytoplasm such that we had to pre-treat the cells with a microtubule-disrupting agent in order to perform the kinetic measurements. The positioning of the hydrophobic side-chain analogues at the very end of already surface-exposed side-chains of BSA was probably crucial to enable interactions of the conjugates with the FG-repeats. Such an extensive protrusion of hydrophobic side-chains into the solvent does not normally occur in soluble proteins, which is why native proteins whose size is above the cut-off limit cannot cross NPCs unassisted. Another advantage to having specifically positioned and structured FG-binding sites is that it provides a means for controlling the interaction between NTRs and the pores through conformational changes induced by ligand binding, e.g., RanGTP. This is required to dissociate certain NTRs from nucleoporins at the pores' edgesCitation59–Citation62 and may play a role in regulating their passage through the central pore channel. Thus despite the fact that surface hydrophobicity is sufficient to provide cargoes with the ability to traverse the NPC, it seems that a more sophisticated design is necessary to allow NTRs to function in the highly crowded and complex environment of the cell.
Abbreviations
| NES | = | nuclear export signal |
| NLS | = | nuclear localisation signal |
| NPC | = | nuclear pore complex |
| NTR | = | nuclear transport receptor |
| Nups | = | nucleoporins, proteins that make up the nuclear pore complex |
Figures and Tables
Figure 1 NPC structure and mediated-transport cycles. NPCs fuse the inner and outer membranes of the nuclear envelope (blue), forming aqueous channels that communicate between the nucleus and the cytoplasm. The vertebrate NPC measures about 120 × 90 nm and is made up of ∼30 different proteins, called nucleoporins or Nups, most of which are present in multiples of eight. Associated with the core scaffold of the assembly are eight, ∼50 nm long filaments that protrude towards the cytoplasm and a massive, fish trap-like structure, termed the nuclear basket, which extends about 50 nm into the nucleoplasm. (Left part) Nuclear import. A protein carrying a nuclear localisation signal binds to an import transport receptor in the cytoplasm. Upon reaching the nucleoplasmic face of the pore, binding of RanGTP to the transport receptor frees the latter from FG-repeats in the pore and dissociates the complex. (Right part) Nuclear export. A ternary export complex is formed between a nuclear export receptor, a nuclear export signal-bearing cargo and RanGTP, which typically increases the affinity of export receptors to their cargo. The complex traffics to the cytoplasmic face of the NPC where it is disassembled and the Ran-bound GTP is concomitantly hydrolysed in a process requiring one of two Ran-binding proteins, called RanBP1 and RanBP2 and Ran's GTPase-activating protein RanGAP1. Due to its small size, Ran can, in principle, cross the NPC by passive diffusion. However, to maintain a steep RanGTP gradient across the NE, the transport of GDP-loaded Ran back to the nucleus, where recharging with GTP takes place, is facilitated by a dedicated import receptor called NTF2.
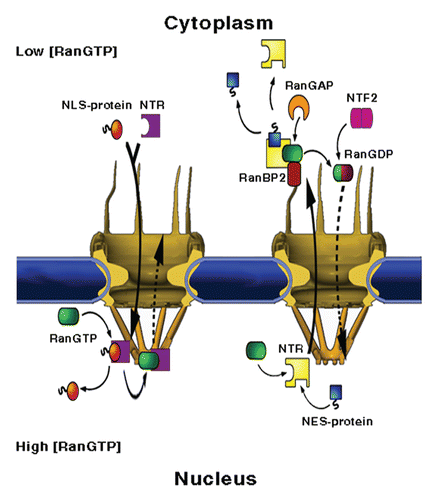
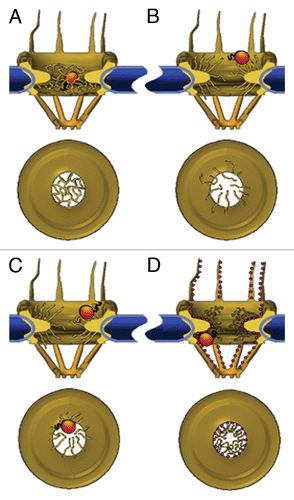
Figure 2 Models of the NPC permeability barrier. (A) Self-interacting FG-repeats form a meshwork within the central pore channel; only molecules that are smaller than the mesh size can go through unassisted. NTRs partition into the meshwork by replacing internal FG-FG contacts with interactions with their hydrophobic patches, virtually ‘dissolving’ into the polymer gel. (B) Unlinked FG-repeats at the edges of the NPCs form polymer brushes that exclude macromolecules through their thermal motion. NTRs permeate this barrier by binding to the FG-repeats, thereby increasing their probability of entry. (C) NTR binding induces collapse of FG-domains, which is reversed by dissociation of the receptor. Transport is afforded by repeated collapse and re-extension events. (D) FG-repeats (shown here as small red spheres) collapse to form a continuous layer extending from the cytoplasmic filaments, through the interior of the central pore channel, to the nuclear filaments. NTRs glide over this surface in a 2D random-walk fashion whereas small molecules pass through an aqueous tube located at the centre of the conductive channel. The space between this tube and the FG-layer in the channel is assumed to be occupied by a loose network of hydrophilic flexible polypeptides, possibly the hydrophilic stretches that connect between the FG-repeats.
Extra View on: Naim B, Zbaida D, Dagan S, Kapon R, Reich Z. Cargo surface hydrophobicity is sufficient to overcome the nuclear pore complex selectivity barrier. EMBO J 2009; 28:2697 - 2705
References
- Paine PL, Moore LC, Horowitz SB. Nuclear envelope permeability. Nature 1975; 254:14 - 17
- Keminer O, Peters R. Permeability of single nuclear pores. Biophys J 1999; 77:217 - 228
- Chook YM, Blobel G. Karyopherins and nuclear import. Curr Opin Struct Biol 2001; 11:703 - 715
- Conti E, Muller CW, Stewart M. Karyopherin flexibility in nucleocytoplasmic transport. Curr Opin Struct Biol 2006; 16:237 - 244
- Cook A, Bono F, Jinek M, Conti E. Structural biology of nucleocytoplasmic transport. Annu Rev Biochem 2007; 76:647 - 671
- Bayliss R, Littlewood T, Stewart M. Structural basis for the interaction between FxFG nucleoporin repeats and importin-beta in nuclear trafficking. Cell 2000; 102:99 - 108
- Bayliss R, Littlewood T, Strawn LA, Wente SR, Stewart M. GLFG and FxFG nucleoporins bind to overlapping sites on importin-beta. J Biol Chem 2002; 277:50597 - 50606
- Morrison J, Yang JC, Stewart M, Neuhaus D. Solution NMR study of the interaction between NTF2 and nucleoporin FxFG repeats. J Mol Biol 2003; 333:587 - 603
- Isgro TA, Schulten K. Binding dynamics of isolated nucleoporin repeat regions to importin-beta. Structure 2005; 13:1869 - 1879
- Liu SM, Stewart M. Structural basis for the highaffinity binding of nucleoporin Nup1p to the Saccharomyces cerevisiae importin-beta homologue, Kap95p. J Mol Biol 2005; 349:515 - 525
- Isgro TA, Schulten K. Cse1p-binding dynamics reveal a binding pattern for FG-repeat nucleoporins on transport receptors. Structure 2007; 15:977 - 991
- Isgro TA, Schulten K. Association of nuclear pore FG-repeat domains to NTF2 import and export complexes. J Mol Biol 2007; 366:330 - 345
- Quan Y, Ji ZL, Wang X, Tartakoff AM, Tao T. Evolutionary and transcriptional analysis of karyopherin beta superfamily proteins. Mol Cell Proteomics 2008; 7:1254 - 1269
- Mattaj IW, Englmeier L. Nucleocytoplasmic transport: the soluble phase. Annu Rev Biochem 1998; 67:265 - 306
- Gorlich D, Kutay U. Transport between the cell nucleus and the cytoplasm. Annu Rev Cell Dev Biol 1999; 15:607 - 660
- Weis K. Regulating access to the genome: nucleocytoplasmic transport throughout the cell cycle. Cell 2003; 112:441 - 451
- Pemberton LF, Paschal BM. Mechanisms of receptor-mediated nuclear import and nuclear export. Traffic 2005; 6:187 - 198
- Steggerda SM, Paschal BM. Regulation of nuclear import and export by the GTPase Ran. Int Rev Cytol 2002; 217:41 - 91
- Kuersten S, Ohno M, Mattaj IW. Nucleocytoplasmic transport: Ran, beta and beyond. Rends Cell Biol 2001; 11:497 - 503
- Terry LJ, Wente SR. Flexible gates: dynamic topologies and functions for FG nucleoporins in nucleocytoplasmic transport. Eukaryotic cell 2009; 8:1814 - 1827
- Alber F, Dokudovskaya S, Veenhoff LM, Zhang W, Kipper J, Devos D, et al. The molecular architecture of the nuclear pore complex. Nature 2007; 450:695 - 701
- Ribbeck K, Gorlich D. Kinetic analysis of translocation through nuclear pore complexes. EMBO J 2001; 20:1320 - 1330
- Ribbeck K, Gorlich D. The permeability barrier of nuclear pore complexes appears to operate via hydrophobic exclusion. EMBO J 2002; 21:2664 - 2671
- Frey S, Richter RP, Gorlich D. FG-rich repeats of nuclear pore proteins form a three-dimensional meshwork with hydrogel-like properties. Science 2006; 314:815 - 817
- Frey S, Gorlich D. A saturated FG-repeat hydrogel can reproduce the permeability properties of nuclear pore complexes. Cell 2007; 130:512 - 523
- Frey S, Gorlich D. FG/FxFG as well as GLFG repeats form a selective permeability barrier with self-healing properties. EMBO J 2009; 28:2554 - 2567
- Rout MP, Aitchison JD, Magnasco MO, Chait BT. Virtual gating and nuclear transport: the hole picture. Trends Cell Biol 2003; 13:622 - 628
- Jovanovic-Talisman T, Tetenbaum-Novatt J, McKenney AS, Zilman A, Peters R, Rout MP, et al. Artificial nanopores that mimic the transport selectivity of the nuclear pore complex. Nature 2009; 457:1023 - 1027
- Lim RY, Huang NP, Koser J, Deng J, Lau KH, Schwarz-Herion K, et al. Flexible phenylalanineglycine nucleoporins as entropic barriers to nucleocytoplasmic transport. Proc Natl Acad Sci USA 2006; 103:9512 - 9517
- Lim RY, Fahrenkrog B, Koser J, Schwarz-Herion K, Deng J, Aebi U. Nanomechanical basis of selective gating by the nuclear pore complex. Science 2007; 318:640 - 643
- Peters R. Translocation through the nuclear pore complex: selectivity and speed by reduction-of-dimensionality. Traffic 2005; 6:421 - 427
- Ma J, Yang W. Three-dimensional distribution of transient interactions in the nuclear pore complex obtained from single-molecule snapshots. Proc Natl Acad Sci USA 107:7305 - 7310
- Yamada J, Phillips JL, Patel S, Goldfien G, Calestagne-Morelli A, Huang H, et al. A bimodal distribution of two distinct categories of intrinsically-disordered structures with separate functions in FG nucleoporins. Mol Cell Proteomics
- Hinshaw JE, Carragher BO, Milligan RA. Architecture and design of the nuclear pore complex. Cell 1992; 69:1133 - 1141
- Akey CW, Radermacher M. Architecture of the Xenopus nuclear pore complex revealed by three-dimensional cryo-electron microscopy. J Cell Biol 1993; 122:1 - 19
- Stoffler D, Feja B, Fahrenkrog B, Walz J, Typke D, Aebi U. Cryo-electron tomography provides novel insights into nuclear pore architecture: implications for nucleocytoplasmic transport. J Mol Biol 2003; 328:119 - 130
- Beck M, Forster F, Ecke M, Plitzko JM, Melchior F, Gerisch G, et al. Nuclear pore complex structure and dynamics revealed by cryoelectron tomography. Science 2004; 306:1387 - 1390
- Goldberg MW, Allen TD. The nuclear pore complex: three-dimensional surface structure revealed by field emission, in-lens scanning electron microscopy, with underlying structure uncovered by proteolysis. J Cell Sci 1993; 106:261 - 274
- Nevo R, Markiewicz P, Kapon R, Elbaum M, Reich Z. High-resolution imaging of the nuclear pore complex by AC scanning force microscopy. Single molecules 2000; 1:109 - 114
- Rout MP, Aitchison JD, Suprapto A, Hjertaas K, Zhao Y, Chait BT. The yeast nuclear pore complex: composition, architecture and transport mechanism. J Cell Biol 2000; 148:635 - 651
- Talcott B, Moore MS. Getting across the nuclear pore complex. Trends Cell Biol 1999; 9:312 - 318
- Ben-Efraim I, Gerace L. Gradient of increasing affinity of importin beta for nucleoporins along the pathway of nuclear import. J Cell Biol 2001; 152:411 - 417
- Allen NP, Huang L, Burlingame A, Rexach M. Proteomic analysis of nucleoporin interacting proteins. J Biol Chem 2001; 276:29268 - 29274
- Pyhtila B, Rexach M. A gradient of affinity for the karyopherin Kap95p along the yeast nuclear pore complex. J Biol Chem 2003; 278:42699 - 42709
- Patel SS, Rexach MF. Discovering novel interactions at the nuclear pore complex using bead halo: a rapid method for detecting molecular interactions of high and low affinity at equilibrium. Mol Cell Proteomics 2008; 7:121 - 131
- Tokunaga M, Imamoto N, Sakata-Sogawa K. Highly inclined thin illumination enables clear single-molecule imaging in cells. Nat Methods 2008; 5:159 - 161
- Naim B, Zbaida D, Dagan S, Kapon R, Reich Z. Cargo surface hydrophobicity is sufficient to overcome the nuclear pore complex selectivity barrier. EMBO J 2009; 28:2697 - 2705
- Patel SS, Belmont BJ, Sante JM, Rexach MF. Natively unfolded nucleoporins gate protein diffusion across the nuclear pore complex. Cell 2007; 129:83 - 96
- Zilman A, Di Talia S, Chait BT, Rout MP, Magnasco MO. Efficiency, selectivity and robustness of nucleocytoplasmic transport. PLoS Comput Biol 2007; 3:125
- Eisele NB, Frey S, Piehler J, Gorlich D, Richter RP. Ultrathin nucleoporin phenylalanine-glycine repeat films and their interaction with nuclear transport receptors. EMBO rep
- Naim B, Brumfeld V, Kapon R, Kiss V, Nevo R, Reich Z. Passive and facilitated transport in nuclear pore complexes is largely uncoupled. J Biol Chem 2007; 282:3881 - 3888
- Danker T, Schillers H, Storck J, Shahin V, Kramer B, Wilhelmi M, et al. Nuclear hourglass technique: an approach that detects electrically open nuclear pores in Xenopus laevis oocyte. Proc Natl Acad Sci USA 1999; 96:13530 - 13535
- Shahin V, Danker T, Enss K, Ossig R, Oberleithner H. Evidence for Ca2+- and ATP-sensitive peripheral channels in nuclear pore complexes. Faseb J 2001; 15:1895 - 1901
- Mazzanti M, Bustamante JO, Oberleithner H. Electrical dimension of the nuclear envelope. Physiol Rev 2001; 81:1 - 19
- Enss K, Danker T, Schlune A, Buchholz I, Oberleithner H. Passive transport of macromolecules through Xenopus laevis nuclear envelope. J Membr Biol 2003; 196:147 - 155
- Kramer A, Ludwig Y, Shahin V, Oberleithner H. A pathway separate from the central channel through the nuclear pore complex for inorganic ions and small macromolecules. J Biol Chem 2007; 282:31437 - 31443
- Kramer A, Liashkovich I, Ludwig Y, Shahin V. Atomic force microscopy visualises a hydrophobic meshwork in the central channel of the nuclear pore. Pflugers Arch 2008; 456:155 - 162
- Feldherr CM, Akin D. The location of the transport gate in the nuclear pore complex. J Cell Sci 1997; 110:3065 - 3070
- Rexach M, Blobel G. Protein import into nuclei: association and dissociation reactions involving transport substrate, transport factors and nucleoporins. Cell 1995; 83:683 - 692
- Gorlich D, Pante N, Kutay U, Aebi U, Bischoff FR. Identification of different roles for RanGDP and RanGTP in nuclear protein import. EMBO J 1996; 15:5584 - 5594
- Shah S, Forbes DJ. Separate nuclear import pathways converge on the nucleoporin Nup153 and can be dissected with dominant-negative inhibitors. Curr Biol 1998; 8:1376 - 1386
- Shah S, Tugendreich S, Forbes D. Major binding sites for the nuclear import receptor are the internal nucleoporin Nup153 and the adjacent nuclear filament protein Tpr. J Cell Biol 1998; 141:31 - 49