Abstract
Fibroblasts derived from Hutchinson-Gilford progeria syndrome (HGPS) patients and dermal cells derived from healthy old humans in culture display age-dependent progressive changes in nuclear architecture due to accumulation of farnesylated lamin A. Treating human HGPS cells or mice expressing farnesylated lamin A with farnesyl transferase inhibitors (FTIs) reverses nuclear phenotypes and extends lifespan. Aging adult Caenorhabditis elegans show changes in nuclear architecture resembling those seen in HGPS fibroblasts, as well as a decline in motility, phenotypes which are also inhibited by the FTI gliotoxin. However, it was not clear whether these effects were due to loss of farnesylation or to side effects of this drug. Here, we used a different FTI, manumycin, or downregulated polyprenyl synthetase with RNAi to test the roles of farnesylation in C. elegans aging. Our results show that the age-dependent changes in nuclear morphology depend on farnesylation. We also demonstrate that inhibition of farnesylation does not affect motility or lifespan, suggesting that the effects of blocking protein prenylation on nuclear morphology could be separated from their effects on motility and lifespan. These results provide further understanding of the role of lamin and farnesylation in the normal aging process and in HGPS.
Introduction
The nuclear lamina is a fibrillar network that is located between the inner nuclear membrane and the peripheral chromatin. It is composed of lamins, integral membrane proteins, transcription factors and signaling proteins. Most nuclear lamina proteins are associated directly or indirectly with lamins, which are nuclear intermediate filament proteins.Citation1,Citation2 The nuclear lamina is required for most nuclear activities, including chromatin organization, cell cycle regulation, nuclear positioning within the cell, DNA replication, RNA Pol II transcription, as well as for modulating master regulatory genes and signaling pathways.Citation3–Citation5 Mutations in human lamin genes, mainly in LMNA, cause over 16 different laminopathies affecting multiple cell types and tissues, including muscle, adipocytes, peripheral nerves, bone and skin.Citation6
One group of laminopathies is comprised of progeroid syndromes, including Hutchinson-Gilford progeria syndrome (HGPS), atypical Werner syndrome and mandibuloacral dysplasia. These diseases are caused by mutations either in LMNA or ZMPSTE24, which encodes one of the prelamin A processing enzymes.Citation7 Most HGPS patients express the mutant lamin A isoform progerin, which is permanently farnesylated.Citation8 With increasing passage number, HGPS fibroblasts in culture accumulate progerin and changes are seen in their nuclear morphology and chromatin organization.Citation9,Citation10 Blocking lamin farnesylation in HGPS fibroblasts by either farnesyl transferase inhibitors (FTIs) or by a combination of statins and aminobisphosphonates restores nuclear morphology and reverses the changes in chromatin organization. In addition, blocking farnesylation in mouse models of constitutive farnesylation improved their growth, body weight, lifespan and bone defects.Citation11–Citation14 These results have led to ongoing clinical trials of statins plus aminobisphosphonates with or without FTI in children with HGPS.
Progerin mRNA and protein tend to accumulate in dermal fibroblast cultures obtained from old healthy individuals.Citation15,Citation16 The progerin accumulation is enriched in binucleated cells with nuclear defects, similar to those seen in HGPS cells.Citation15 Progerin is also found in skin biopsies from healthy individuals and appears in higher levels in old people.Citation16 This suggests that progerin plays a role in the normal aging process as well as HGPS.
In the nematode Caenorhabditis elegans, somatic cells stop dividing in the adult stage, making this animal a good model for studying aging in non-proliferating tissues.Citation17 Lamins are essential for normal longevity in C. elegans, as reduced lamin activity at the adult stage shortens lifespan accompanied by accelerated aging phenotypes.Citation19 In most non-neuronal cell types, the nuclear envelope (NE) undergoes progressive and stochastic age-dependent alterations, resembling changes observed in HGPS fibroblasts, which can be defined as one of three phases.Citation18 At an early age, most nuclei appear round or slightly ovule, with lamin and the inner nuclear membrane protein emerin evenly distributed around the periphery (Phase I); as worms age most somatic nuclei become convoluted and lobulated, with lamin aggregates in foci (Phase II). Over time, there is an increase in nucleoplasmic lamin, a decrease in nuclear peripheral lamin and nuclei become abnormally shaped with extensive stretching and fragmentation. These morphological changes are accompanied by loss of peripheral heterochromatin and accumulation of chromatin away from the nuclear periphery (Phase III).Citation18
In a previous study, we used the FTI gliotoxin to investigate the effects of inhibiting lamin farnesylation on aging in adult C. elegans cells.Citation20 This drug caused a marked improvement in nuclear shape, amelioration of the age-related motility decline, but with no effect on lifespan. However, it was unclear if these phenotypes could be attributed to lamin farnesylation or to other cytotoxic effects of this drug. Here we investigate an additional FTI, manumycin, as well as the global inhibition of farnesylation by downregulation of polyprenyl synthetase. We show that while restoration of nuclear shape is a result of inhibition of farnesylation, improved age-dependent motility is probably a side effect of FTIs. Furthermore, blocking farnesylation can help maintain normal chromatin distribution in nuclei of aging animals, but is insufficient to extend lifespan.
Results
Manumycin rescues age-related changes in nuclear morphology.
As worms age, the NE loses its round shape and becomes lobulated and convoluted and Ce-lamin aggregates at the nuclear periphery. The nuclear deterioration can be partially delayed in some long-lived strains.Citation18 We had previously shown that feeding C. elegans with the FTI gliotoxin can inhibit the age-related deterioration of NE shape: nuclei from gliotoxin-treated worms showed fewer lobules and aggregations and retained their circular shape.Citation20 We repeated these experiments using a different FTI, manumycin, which was previously characterized in C. elegans larval development,Citation25 in order to determine the general effects of FTIs on NE structure. Transgenic young adult worms were treated with 63–500 nM of manumycin and the shape of the nuclei was monitored in live animals expressing GFP::lamin or GFP::emerin in a wild-type background. Manumycin at concentrations of 125 nM and 250 nM reduced the NE lobulation of these worms at days 4 (, upper part) and 6 (, lower part) of adulthood, supporting the previous conclusion that the shape rescue by FTIs is likely to result from inhibition of farnesylation. 500 nM manumycin reduced the NE lobulation, but was later toxic to the animals and they died before day 6 of adulthood. The ability to inhibit nuclear morphology changes was more prominent at high manumycin concentrations and to a lesser extent than gliotoxin.Citation20
Manumycin can rescue age-related reduction in motility.
A decline in movement is a hallmark of aging, which is well characterized in C. elegans.Citation26,Citation27 As gliotoxin improves motility in aging C. elegans,Citation20 we investigated if this can be also achieved with manumycin. Wild-type animals were transferred at day 1 of adulthood to plates containing 125 or 250 nM manumycin and motility was assayed as the average velocity on agar plates after 2, 3, 6 and 8 days of treatment at 20°C (). Until day 8 of adulthood, manumycin at both concentrations significantly improved the average velocity of animals (p < 0.01 for manumycin treated animals vs. control). By day 8 of adulthood there was a significant reduction in motility in both wildtype and manumycin-treated animals (p < 0.01), but the manumycin-treated animals still had higher average velocity. However, by day 11 of adulthood there was no significant difference in the average velocity between control and treated worms, which hardly moved at all tested concentrations (data not shown). We concluded that the ability of manumycin to improve the age-related reduction in motility is temporal and applies mostly to early days of adulthood.
Manumycin shortens lifespan in a dose-dependent manner.
We next examined the effect of manumycin on average lifespan. Treating wild-type C. elegans with manumycin at all tested concentrations shortened the average lifespan of the worms in a dose-dependent manner (), demonstrating a toxic effect of this drug and making it impossible to draw conclusions on the effects of manumycin on lifespan.
Downregulating polyprenyl synthetase rescues the NE aging phenotype.
Previous studies have shown that in the absence of farnesyl transferase, geranylgeranyl transferase can attach a geranylgeranyl moiety to the cysteine of the C-terminus CAAX motif of lamin, thus partially restoring the function of farnesylation to titrate lamin A to the NE.Citation14 In order to inhibit both farnesylation and geranylgeranylation, aminobisphospanates with statins were used to block the production of farnesyl-PP needed both for prenylation reactions.Citation14 However, Zoledronate together with pravastatin at different concentrations failed to affect farnesylation in C. elegans, as judged by the multivulva assay for Ras farnesylationCitation25 (data not shown). To directly inhibit the production of farnesyl-PP, we generated a dsRNA construct against polyprenyl synthetase (R06C1.2; fdps-1(RNAi)) and used it to efficiently downregulate fdps-1 mRNA in adult C. elegans (Sup. Fig. 1). Animals grown on bacteria expressing fdps-1(RNAi) showed a significant inhibition of the age-dependent changes in NE shape: the aging nuclei of the fdps-1(RNAi)-treated animals had no lobulation or membrane proliferation (). The fdps-1(RNAi)-treated nuclei contained many lamin aggregates mostly at the nuclear periphery. fdps-1RNAi also partially rescued the multivulva phenotype in Ras mutated worms (data not shown). We concluded that preventing all prenylation reactions is sufficient to inhibit lobulation and nuclear membrane proliferation and to maintain a nuclear shape that is characteristic to young animals.
Downregulation of polyprenyl synthetase inhibits peripheral chromatin redistribution in aging C. elegans nuclei.
In C. elegans muscle cells, dense DNA staining by Hoechst 33258, which probably represent regions of heterochromatin, is located mainly at the nuclear periphery ( and day 6). As the animals age, the peripheral staining of DNA is lost ( and day 13, L4440). Interestingly, downregulation of polyprenyl synthetase by fdps-1(RNAi) maintained the DNA staining at the nuclear periphery ( and day 13, fdps-1).
Age dependent decline in motility is not affected by prenylation.
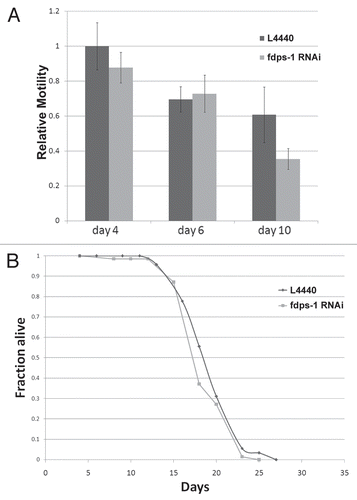
The pharmacological inhibitors of farnesylation, gliotoxin and manumycin, partially rescued the age-dependent deterioration in motility. However, it was not clear whether this rescue is due to the inhibition of farnesylation itself or to a different (side) effect of these drugs. We therefore scored the motility of animals as they aged following downregulation of polyprenyl synthetase by fdps-1 dsRNA. Interestingly, the motility of the fdps-1(RNAi)-treated wild-type animals at days 2, 4 and 6 was similar to control animals treated with an empty RNAi vector (). We concluded that the effect of FTIs on the age-related reduction in motility is probably not due to inhibition of farnesylation.
Inhibition of prenylation has no effect on lifespan.
We next wanted to examine whether the inhibition of nuclear membrane lobulation and invagination correlates with a change in the average lifespan. As shown in , downregulation of polyprenyl synthetase with fdps-1 dsRNA had no effect on the average lifespan of wild-type animals, similar to the treatment of C. elegans with gliotoxin.Citation20 We concluded that both drug and genetic manipulation of prenylation do not extend lifespan in C. elegans.
Discussion
Protein farnesylation causes nuclear lobulation and membrane proliferation.
Accumulation of progerin, a permanently farnesylated form of lamin A, in nuclei of HGPS patients or in nuclei of normally aging cells leads to nuclear deformation, change in heterochromatin distribution and aging diseases. Mutations in the lamin A processing enzyme ZMPSTE24, which also increase the amount of farnesylated prelamin A, also cause similar phenotypes.Citation28–Citation30 Inhibition of farnesylation by FTIs or by statins plus aminobisphosphonates in HGPS fibroblasts, HeLa cells expressing progerin, ZMPSTE24 mutant cells expressing prelamin A, mice cells expressing farnesylated lamin A or mice cells expressing mutant ZMPSTE24 display decreased lobulation, membrane invagination and chromatin phenotypes.Citation13,Citation14,Citation31,Citation32 These effects of farnesylation on nuclear morphology are probably conserved in evolution, since in C. elegans inhibition of farnesylation either by FTIs or by genetic downregulation of polyprenyl synthetase inhibited both the age-dependent phenotypes of nuclear deformation and the redistribution of heterochromatin. The change in nuclear shape could be explained at least in part by the role of farnesylation of nuclear envelope proteins in inducing membrane proliferation, as was shown for overexpression of lamins B1 and B2 in Xenopus oocytes,Citation33,Citation34 overexpression of farnesylated lamins in COS-7 cells or in zebrafish embryos,Citation35 or expression of the farnesylated inner nuclear membrane protein kugelkern in Drosophila embryos.Citation36 Taken together, these results show that prenylation of lamin probably plays a major role in membrane proliferation and invaginations in both HGPS and normal aging nuclei.
FTIs can delay the age-dependent motility phenotypes.
In aging adult C. elegans, body movement slows down and ultimately ceases altogether. Treating adult C. elegans with gliotoxinCitation20 or manumycin (this study) delayed, but did not inhibit, the age-dependent reduction in motility. However, at later days of adulthood the motility of the FTIs-treated animals was similar to that of the control animals. These results suggest that the reduction in motility of aging animals has probably two phases, one dependent and one independent of these drugs. An alternative explanation is that the response of muscle cells to FTIs is lost in the aging animals. Interestingly, downregulation of polyprenyl synthetase had no effect on the age-dependent reduction in motility; at all tested ages the fdps-1(RNAi) and control adult animals showed similar motility. These results demonstrate that the effect of gliotoxin and manumycin on the animal motility during earlier days is caused by activity other then farnesyl transferase inhibition.
The effect of farnesylation on the average lifespan of C. elegans.
Manumycin had toxic effects on C. elegans that led to reduction of the average lifespan. This result is in contrast to gliotoxin, which had no effects on the average lifespan.Citation20 Downregulation of polyprenyl synthetase at the adult stages had no effect on the average lifespan of the C. elegans, similar to gliotoxin. While gliotoxin or manumycin could potentially affect the average lifespan by slowing the reduction in motility in aging animals,Citation37 this was not the case for the fdps-1(RNAi) treatment. Besides inhibiting lamin farnesylation, FTIs inhibit farnesylation of many other proteins and can have multiple side effects. For example, gliotoxin is an inhibitor of the chymotrypsin-like activity of the 20S proteasome and both manumycin and gliotoxin are antibiotics, affecting the bacteria consumed by C. elegans.Citation38 It is therefore not clear if the inability to extend lifespan by FTIs resulted from their deleterious side effects.
Studies in mouse models for HGPS have shown life extension following inhibition of farnesylation.Citation14,Citation39 The lack of life extension in C. elegans following inhibition of farnesylation by FTI or by downregulation of polyprenyl synthetase might be due to the fact that C. elegans has no bona fide A-type lamin. Also, FTIs were so far tested only on HGPS models, but not on normally aging animals. Finally, FTIs and genetic inhibition of farnesylation could affect proteins that affect lifespan that are not conserved between C. elegans and human.
Materials and Methods
C. elegans strains.
Strain maintenance and manipulations were performed under standard conditions as described.Citation17 The following strains were used: wild-type (N2); MT2124, a non-lethal let-60 mutation (n1046) resulting in a multivulva phenotype; a transgenic strain expressing GFP::Ce-lamin under the promoter of baf-1 (baf-1p-gfp::lmn-1),Citation21 YG301; and a transgenic strain expressing Ce-emerin::GFP under the promoter of lmn-1 (lmn-1p-emr-1::gfp), YG002. All strains are integrated and were generated by bombardment, followed by three out-crosses.Citation22
Fluorescence microscopy.
Live fluorescent images were acquired with the Lieca SP5 confocal microscope and a 63X/1.4 oil immersion objective. Worms were paralyzed with 1 mM levamisole. For live DNA imaging, YG002 worms were washed with 1 mg/ml Hoechst 33258 in M9 for 5 min, followed by a brief wash with M9.
Motility assays.
Multiple N2 animals were recorded on NGM agar plates for 5 min intervals at 10 frames per second. At least 10 worms were used for every treatment and the experiments were repeated at least twice. Motility was analyzed using Worm-Tracker for image tracking.Citation23 Recording was made using Dino-Eye (Dino-Lite Microscope, NY) on a Nikon SZM800 binocular or on Axiocam CCD camera mounted on an Olympus MVX10 microscope. p-values were calculated using unpaired equal distribution t-test.
Lifespan assays.
The protocol for measuring lifespan was slightly modified from described.Citation24 N2 animals were synchronized by bleach and grown on nematode growth medium (NGM) at 20°C until they reached young adult stage. For each experiment, about 100 adult animals were transferred to 3–5 NGM plates containing 0.05 mg/ml 5-fluoro 2O-deoxyuridine (FUDR), to prevent growth of progeny. The desired amount of manumycin (Sigma) was added to plates seeded with OP50. Fdps-1(RNAi) or L4440 (empty vector) expressed in bacteria were seeded on feeding plates containing 50 µg/ml ampicillin and 120 µg/ml IPTG. Animals were considered dead when they no longer responded to gentle prodding with a platinum wire. Scoring was performed every other day. Animals that exploded or crawled out of the plate were censored from the experiments. Previous work showed no significant effect of FUDR on lifespan.Citation19 For all lifespan experiments, assays were repeated at least twice.
Figures and Tables
Figure 1 Manumycin alters nuclear morphology. GFP::Ce-lamin in live control and manumycin treated worms at days 4 (upper part) and 6 (lower part) of adulthood. C. elegans were treated with 125, 250 and 500 nM of manumycin and grown at 20°C. Nuclei in worms treated with manumycin show fewer convolutions compared to nuclei from untreated worms. Bar = 10 microns and applies to all parts.
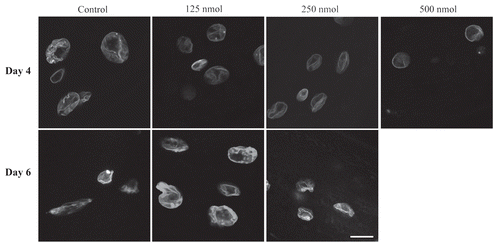
Figure 2 Manumycin affects motility and is toxic to C. elegans. (A) Relative motility of control and manumycin-treated worms. Black bars indicate control; dark gray bars 125 nM manumycin and light gray bars 250 nM of manumycin. Y-axis shows relative average motility to control worms at day 1 of adulthood. (B) Survival plot of control and manumycin treated animals. The amount of manumycin is indicated next to each graph. The animal lifespan was shortened with increased doses of manumycin.
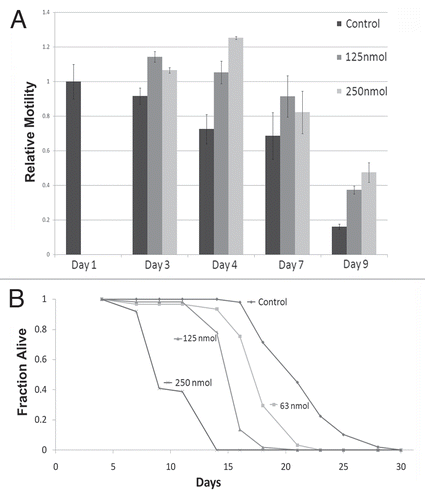
Figure 3 Nuclei retain their round morphology following polyprenyl synthetase downregulation by fdps-1(RNAi). (A) Animals were treated with either the empty vector L4440 (EV) or with fdps-1(RNAi) starting at the young adult stage (day 1). GFP::Ce-lamin was used to view changes in nuclear and nuclear envelope shapes. The fdps-1(RNAi)-treated nuclei showed some lamin aggregations at the nuclear periphery, but no lobulation. Bars = 10 microns.
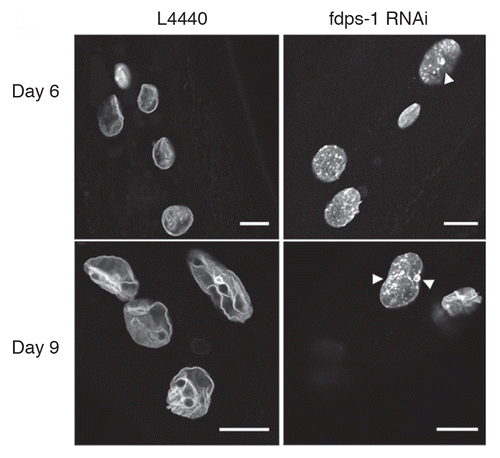
Figure 4 Downregulation of polyprenyl synthetase by fdps-1(RNAi) blocks the redistribution of chromatin in nuclei of aging cells. Animals were treated with either L4440 vector (EV) or with fdps-1(RNAi) starting at the young adult stage (day 1). Ce-emerin::GFP and Hoechst DNA staining were used to view changes in nuclear envelope shapes and chromatin distribution. fdps-1(RNAi)-treated worms show diminished chromatin mislocalization at old age. In the merge parts green = lamin; blue = DNA. Bars = 10 microns.
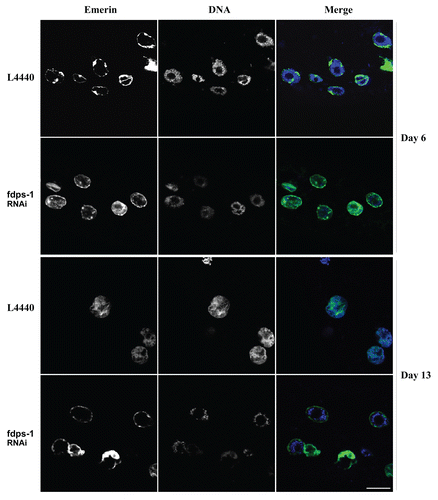
Figure 5 Downregulation of polyprenyl synthetase by fdps-1(RNAi) has no significant effect on motility and lifespan. (A) Relative motility of control (dark gray) and fdps-1(RNAi) (light gray)-treated worms. (B) Survival plot of control- and fdps-1(RNAi)-treated animals. The black line indicates control and the light gray line fdps-1(RNAi).
Additional material
Download Zip (66.5 KB)Acknowledgements
We thank Dr. Erin Bank for excellent comments. This study was funded by grants from the Israel Science Foundation, USA-Israel Binational Science Foundation (BSF), German-Israel Foundation (GIF), the Legacy (Morasha) fund of the Israeli Science Foundation and the Muscular Dystrophy Association (MDA). Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources (NCRR).
References
- Stuurman N, Heins S, Aebi U. Nuclear lamins: their structure, assembly and interactions. J Struct Biol 1998; 122:42 - 66
- Herrmann H, Bär H, Kreplak L, Strelkov SV, Aebi U. Intermediate filaments: from cell architecture to nanomechanics. Nat Rev Cell Mol Biol 2007; 8:562 - 573
- Goldman RD, Gruenbaum Y, Moir RD, Shumaker DK, Spann TP. Nuclear lamins: building blocks of nuclear architecture. Genes Dev 2002; 16:533 - 547
- Gruenbaum Y, Margalit A, Goldman RD, Shumaker DK, Wilson KL. The nuclear lamina comes of age. Nat Rev Mol Cell Biol 2005; 6:21 - 31
- Boban M, Braun J, Foisner R. Lamins: ‘structure goes cycling’. Biochem Soc Trans 2010; 38:301 - 306
- Worman HJ, Ostlund C, Wang Y. Diseases of the nuclear envelope. Cold Spring Harb Perspect Biol 2010; 2:000760
- Ramírez CL, Cadiäanos J, Varela I, Freije JM, López-Otín C. Human progeroid syndromes, aging and cancer: new genetic and epigenetic insights into old questions. Cell Mol Life Sci 2007; 64:155 - 170
- Dechat T, Pfleghaar K, Sengupta K, Shimi T, Shumaker DK, Solimando L, et al. Nuclear lamins: major factors in the structural organization and function of the nucleus and chromatin. Genes Dev 2008; 22:832 - 853
- Goldman RD, Shumaker DK, Erdos MR, Eriksson M, Goldman AE, Gordon LB, et al. Accumulation of mutant lamin A causes progressive changes in nuclear architecture in Hutchinson-Gilford Progeria Syndrome 2004; 101:8963 - 8968
- Scaffidi P, Misteli T. Reversal of the cellular phenotype in the premature aging disease Hutchinson-Gilford progeria syndrome. Nat Med 2005; 11:440 - 445
- Fong LG, Frost D, Meta M, Qiao X, Yang SH, Coffinier C, Young SG. A protein farnesyltransferase inhibitor ameliorates disease in a mouse model of progeria. Science 2006; 311:1621 - 1623
- Meta M, Yang SH, Bergo MO, Fong LG, Young SG. Protein farnesyltransferase inhibitors and progeria. Trends Mol Med 2006; 12:480 - 487
- Yang SH, Meta M, Qiao X, Frost D, Bauch J, Coffinier C, et al. A farnesyltransferase inhibitor improves disease phenotypes in mice with a Hutchinson-Gilford progeria syndrome mutation. J Clin Invest 2006; 116:2115 - 2121
- Varela I, Pereira S, Ugalde AP, Navarro CL, Suárez MF, Cau P, et al. Combined treatment with statins and aminobisphosphonates extends longevity in a mouse model of human premature aging. Nat Med 2008; 14:767 - 772
- Cao K, Capell BC, Erdos MR, Djabali K, Collins FS. A lamin A protein isoform overexpressed in Hutchinson-Gilford progeria syndrome interferes with mitosis in progeria and normal cells. Proc Natl Acad Sci USA 2007; 104:3939 - 3954
- McClintock D, Ratner D, Lokuge M, Owens DM, Gordon LB, Collins FS, et al. The mutant form of lamin A that causes Hutchinson-Gilford progeria is a biomarker of cellular aging in human skin. PLoS One 2007; 2:1269
- Brenner S. The genetics of Caenorhabditis elegans. Genetics 1974; 77:71 - 94
- Haithcock E, Dayani Y, Neufeld E, Zahand AJ, Feinstein N, Mattout A, et al. Age-related changes of nuclear architecture in Caenorhabditis elegans. Proc Natl Acad Sci USA 2005; 102:16690 - 16695
- Haithcock E, Dayani Y, Neufeld E, Zahand AJ, Feinstein N, Mattout N, et al. Age-related changes of nuclear architecture in Caenorhabditis elegans. Proc Natl Acad Sci USA 2005; 102:16690 - 16695
- Bar DZ, Neufeld E, Feinstein N, Gruenbaum Y. Gliotoxin reverses age-dependent nuclear morphology phenotypes, ameliorates motility, but fails to affect lifespan of adult Caenorhabditis elegans. Cell Motil Cytoskeleton 2009; 66:791 - 797
- Margalit A, Neufeld E, Feinstein N, Wilson KL, Podbilewicz B, Gruenbaum Y. Barrier to autointegration factor blocks premature cell fusion and maintains adult muscle integrity in C. elegans. J Cell Biol 2007; 178:661 - 673
- Wiesel N, Mattout A, Melcer S, Melamed-Book N, Herrmann H, Medalia O, et al. Laminopathic mutations interfere with the assembly, localization and dynamics of nuclear lamins. Proc Natl Acad Sci USA 2008; 105:180 - 185
- Ramot D, Johnson BE, Berry TLJ, Carnell L, Goodman MB. The Parallel Worm Tracker: a platform for measuring average speed and drug-induced paralysis in nematodes. PLoS One 2008; 3:2208
- Lee SS, Lee RY, Fraser AG, Kamath RS, Ahringer J, Ruvkun G. A systematic RNAi screen identifies a critical role for mitochondria in C. elegans longevity. Nat Genet 2003; 33:40 - 48
- Hara M, Han M. Ras farnesyltransferase inhibitors suppress the phenotype resulting from an activated ras mutation in Caenorhabditis elegans. Proc Natl Acad Sci USA 1995; 92:3333 - 3337
- Merideth MA, Gordon LB, Clauss S, Sachdev V, Smith AC, Perry MB, et al. Phenotype and course of Hutchinson-Gilford progeria syndrome. N Engl J Med 2008; 358:592 - 604
- Herndon LA, Schmeissner PJ, Dudaronek JM, Brown PA, Listner KM, Sakano Y, et al. Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature 2002; 419:808 - 814
- Young SG, Fong LG, Michaelis S, Prelamin A. Zmpste24, misshapen cell nuclei and progeria—New evidence suggesting that protein farnesylation could be important for disease pathogenesis. J Lipid Res 2005; 46:2531 - 2558
- Navarro CL, Cau P, Lévy N. Molecular bases of progeroid syndromes. Hum Mol Genet 2006; 2:151 - 161
- Broers JL, Ramaekers FC, Bonne G, Yaou RB, Hutchison CJ. Nuclear lamins: laminopathies and their role in premature ageing. Physiol Rev 2006; 86:967 - 1008
- Mallampalli MP, Huyer G, Bendale P, Gelb MH, Michaelis S. Inhibiting farnesylation reverses the nuclear morphology defect in a HeLa cell model for Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci USA 2005; 102:14416 - 14421
- Yang SH, Bergo MO, Toth JI, Qiao X, Hu Y, Sandoval S, et al. Blocking protein farnesyltransferase improves nuclear blebbing in mouse fibroblasts with a targeted Hutchinson-Gilford progeria syndrome mutation. Proc Natl Acad Sci USA 2005; 102:10291 - 10296
- Ralle T, Grund C, Franke WW, Stick R. Intranuclear membrane structure formations by CaaX-containing nuclear proteins. J Cell Sci 2004; 117:6095 - 6104
- Goldberg MW, Huttenlauch I, Hutchison CJ, Stick R. Filaments made from A- and B-type lamins differ in structure and organization. J Cell Sci 2008; 121:215 - 225
- Prufert K, Vogel A, Krohne G. The lamin CxxM motif promotes nuclear membrane growth. J Cell Sci 2004; 117:6105 - 6116
- Brandt A, Papagiannouli F, Wagner N, Wilschbrauninger M, Braun M, Furlong EE, et al. Developmental control of nuclear size and shape by Kugelkern and Kurzkern. Curr Biol 2006; 16:543 - 552
- Collins JJ, Huang C, Hughe S, Kornfeld K. WormBook. The measurement and analysis of age-related changes in Caenorhabditis elegans. The C. elegans Research Community, WormBook 2008; DOI/101895/wormbook11371, http://wwwwormbookorg
- Kroll M, Arenzana-Seisdedos F, Bachelerie F, Thomas D, Friguet B, Conconi M. The secondary fungal metabolite gliotoxin targets proteolytic activities of the proteasome. Chem Biol 1999; 6:689 - 698
- Young SG, Meta M, Yang SH, Fong LG. Prelamin a farnesylation and progeroid syndromes. J Biol Chem 2006; 281:39741 - 39745