Abstract
The transcription machinery in the eukaryotic nucleus generates messenger RNA molecules that translocate through the nucleoplasm, anchor to a nuclear pore, and find their way out into the cytoplasm. The dynamic aspects of these steps in the expression pathway were examined in order to understand the kinetic time-frames of gene activation and message dissemination. Utilizing live-cell imaging and tracking of single mRNPs containing different sized mRNAs and varying numbers of introns and exons, it was possible to quantify the temporal and spatial characteristics of the nucleoplasmic travels of mRNPs as well as the kinetics of translocation through the nuclear pore.
Step-by-Step Along the Gene Expression Pathway
The path of gene expression begins with a signal reaching the promoter of a gene in the nucleus and ends in the production of a specific protein product in the cytoplasm. A variety of signals can converge on a gene leading to the binding of a range of specific transcription factors. For gene activation, the end result at the promoter is always the same, namely the assembly of the basal transcription machinery and the recruitment of RNA polymerase II. Appropriate phosphorylation modifications on the C-terminal domain (CTD) of the polymerase act as the “starter” for this enzyme engine, and send the polymerase shooting down the gene to transcribe a nascent chain of pre-mRNA.Citation1,Citation2 Co-transcriptional RNA processing events begin as the pre-mRNA molecule starts to emerge from the polymerase.Citation3,Citation4 First, the 5′-end of the pre-mRNA is capped by the capping enzymes.Citation5 Then, intronic and exonic sequences in the pre-mRNA are identified by splicing factors. This results in the assembly of the spliceosomal machinery on the nascent transcript and the splicing out of introns and ligation of exons.Citation6 Exon-junction complex (EJC) proteins are then deposited on the newly-made exon-exon junctions.Citation7 These assist in the recruitment of export factors that will render the mRNA competent for export through the nuclear pore complex (NPC).Citation8,Citation9 Other proteins, mainly those of the hnRNP family, coat the mRNA molecule thus forming an mRNP.Citation10 Towards the end of the gene, 3′-end processing factors trim the pre-mRNA and finally the mature mRNP and the polymerase separate.Citation11 Now, the mRNP can begin its travels towards the nuclear pore. The nucleus contains large chromosomes and several nuclear bodies, and presumably the large mRNP will have to find its way between these entanglements of DNA. When finally reaching the NPC, the mRNP should contain the correct “keys” that will allow it to pass through the “gate” into the nucleoplasm.Citation12 The 5′-end of the mRNA leads the way through the NPC and emerges in the cytoplasm where it will encounter ribosomes.Citation13,Citation14 Only mRNAs that passed the quality control checkpoints, which were performed both in the nucleus and in the cytoplasm, will be translated and will produce a functional protein. Since mRNAs must be degraded as a means of regulating gene expression, several mechanisms of mRNA decay ensure the correct turnover rates of the mRNA.Citation15,Citation16
Numerous questions come to mind regarding the kinetic aspects of the nuclear portion of this pathway, which we will categorically divide into three main nuclear phases: transcription, nucleoplasmic transport and NPC export.
Transcription: What is the rate (speed) at which the mRNA is being transcribed? Can these rates change while the polymerase is moving along the gene? How does the time-frame of transcription relate to the time-frame of downstream processes?
Nucleoplasmic transport: What is the biophysical mechanism driving the mRNA from gene to NPC? How long does it take an mRNP to reach the NPC? Does the mRNP find the shortest path out? Does the size of the mRNA matter? Are the kinetics affected if the mRNAs underwent splicing or not? Where exactly in the nuclear landscape do the mRNPs travel, and is the whole nucleoplasmic space available for mRNP movement? Does the mRNP interact with the chromatin as it moves along? Are there “rest-stops” along the way in which the mRNP is further processed?
NPC export: Is there a queue of mRNPs waiting to move through the NPC? Does binding to the NPC necessarily mean that export will occur? How fast is the export of an mRNP through the pore? What is the kinetic mechanism of mRNP passage through the pore? The diameter of some mRNPs are larger than the inner channel of the NPC—when and how do they restructure?
Major strides made in the field of live-cell imaging provide powerful tools for examining these questions on a kinetic level.Citation17 Fluorescent tagging of DNA, mRNA and protein molecules simultaneously, in the same cell, allow us to follow the various steps delineated above within their natural environment.Citation18 Specific genes and their products can be followed in real time.Citation19 Amazing developments in hardware such as fluorescence microscopes, cameras and light sources, provide improved temporal and spatial resolution that are required for following single cells. On the other hand, by concentrating on single cells and single molecules we tend to lose the perspective of the whole population of cells. Therefore, in parallel, the development of genome-wide molecular techniques allows for a broad outlook on gene expression. These provide data and analysis that complement live-cell imaging studies. In this review we will give a brief account of the recent studies that have shed light on the kinetic aspects of the gene expression pathway.
Cell Systems for Following Transcription in Living Cells
The output of the transcriptional process is an mRNA molecule, and therefore in order to detect mRNA transcription as it occurs in real time, a specific fluorescent tag is required. Since direct fluorescent labeling of transcribed mRNA in living cells is difficult, indirect methods have been devised. One of these approaches utilizes a repeated sequence in the mRNA (MS2 binding site, MBS) and its cognate RNA-binding protein (MS2 capsid protein; MS2-CP), originating from bacteriophage, as a means for tagging mRNA in vivo. The MS2 sequence is added as a repeating sequence (typically 24 MBS repeats for mammalian cells) into the 3′UTR of the gene. When this sequence is transcribed, the repeats fold into stem-loop structures that act as high affinity binding sites for the MS2-CP (). Fluorescently tagged MS2-CP (e.g., GFP-MS2-CP) binds to the mRNA as it is being transcribed. In this manner, each mRNA molecule is coated with an average of 30 GFP molecules, in addition to the typical mRNP/hnRNP proteins.Citation20 This renders the mRNP bright over the background of nuclear fluorescence and allows for the detection of single mRNPs in living cells.Citation21 This tagging system was first described in S. cerevisae where the dynamics of single mRNP granules in living yeast cells were demonstrated.Citation22 This technique has been used successfully for following mRNAs in a variety of species, for instance in E. coli,Citation23,Citation24 Drosophila,Citation25,Citation26 the social amoeba DictyosteliumCitation27,Citation28 and mammalian cells.Citation20,Citation21,Citation29,Citation30
The Timeframe of Transcription
The MS2 tagging system was introduced into a model gene that was previously used for detecting DNA and protein in the same cell.Citation31 This new gene construct finally made possible the visualization of the actual gene integration locus,Citation32 the production of the mRNA, and the protein product, all simultaneously in the same cell.Citation33 Since the gene of interest was stably integrated into a human cell line and formed a tandem array containing 200 copies of the same gene, it was possible to detect the site of transcription over the background of the rest of the nucleus. The gene was under inducible control (Tet-On), and when induced to transcribe it produced a prominent fluorescent transcription site in which the transcribed mRNAs could be detected.
The fluorescent signal at the site of transcription depicted a steady state consisting of the generation of new transcripts together with the release of mature mRNAs. Therefore, no obvious fluctuations in fluorescence could be detected or used for measurements of transcription. In order to follow the phase of transcriptional elongation, the fluorescent signal (YFP-MS2-CP) was photobleached using the fluorescence recovery after photobleaching (FRAP) method, and the recovery of YFP-MS2-CP fluorescence was monitored over time.Citation29 The recovery of the YFP signal was due to the generation of new MS2 stem-loops in the newly transcribed mRNAs and their coating by YFP-MS2 proteins. In other words, the rates of fluorescence recovery are a depiction of the enzymatic rates of RNA polymerase II transcription.Citation34 Using mathematical modeling of these data a transcriptional rate of 4.3 kb/min was extracted.Citation29 This was quite surprising since many biochemical measurements had measured Pol II rates ranging between 1–2 kb/min.Citation35–Citation38 The live-cell data shed light on another transcription-linked process. The kinetic data revealed that engaged polymerases could enter a paused state while still engaged on the gene and then resume transcription.Citation29 While the biochemical measurements offered a speed consisting of an averaging between transcription and pausing, the live-cell data provided the ability to “zoom-in” on the transcriptional process and extract both of these kinetics. Moreover, this is an advantage of the tandem array system—since many identical genes are working at the same time it is possible to identify different kinetics occurring simultaneously. This resolution of detecting transcription and pausing cannot be obtained when examining single genes.Citation39 Interestingly, RNA Pol I transcription rates in the natural tandem rDNA array system of the nucleolus were also significantly higher (5.7 kb/min) than previous measurements.Citation40
Still, one might wonder whether the higher rate of Pol II transcription was specific for this artificial gene. Other tandem array experimental systems have since been tested. HIV reporters containing MS2 sequence repeats showed a rate of ∼2 kb/min.Citation30 In a system where a β-actin-MS2 gene was used the rates were found to be 3.3 kb/min.Citation41 But not only live-cell data point in the direction of rapid transcription. An early estimation using radioactive labeling concluded that Pol II rates range from 3–6 kb/min.Citation42 More recent studies performed by either real-time PCR or with microarrays have shown rates of 3–4 kb/min.Citation43–Citation45 These studies suggest that these speeds of transcription are rather constant along the genome no matter whether the polymerase is moving through exonic or intronic regions.Citation45 Still, elegant studies have shown that slowing down of the polymerase at certain introns can affect the outcome of alternative splicing patterns.Citation46,Citation47 Altogether, the first kinetic phase in the mRNA expression pathway is dependent on the speed of the polymerase and the length of the gene sequence. Since the average size of a human gene, including introns, is in the 10–15 kb range, this would mean that the average time-frame of transcription is found in the 3–5 min range.
RNA on the Go
It is well established that the translocation of mRNAs in the nucleus is based on diffusion.Citation48 Tagging of mRNAs in living cells was accomplished with a variety of tags. Originally, the entire population of polyadenylated mRNAs was followed using oligo-dT probes that hybridized with the polyA tails of all mRNAs.Citation49 Such fluorescent probes lit up the whole nucleus and provided diffusion measurements for all mRNA species, large and small. Subsequently, fluorescently-caged oligo-dT probes allowed for photoactivation of mRNAs in only a small region of the nucleus, thereby obtaining better resolution of mRNA dispersal throughout the nucleus.Citation50 These measurements demonstrated diffusion as the prominent mechanism for nucleoplasmic RNA movement, in agreement with previous studies in fixed cells.Citation51,Citation52 The advent of GFP-fusions led to an elegant study in which all polyA tails were tagged with a fluorescent version of the polyA-binding protein 2 (PABP2).Citation53 Using both a wild-type and mutant version of PABP2 that cannot bind mRNA, it was shown that indeed nuclear mRNA moves by diffusion.
The MS2 mRNA-tagging system provided the first opportunity to follow a single species of an mRNA on the single mRNP level.Citation21 Single particle tracking of mRNPs showed that they traveled by diffusion. Constrained diffusion was identified as well, and was assumed to be as a result of structural hindering by the chromatin environment (). Depletion of energy (ATP) levels in the cell proved that mRNP movement per se was not dependent on ATP, and therefore would not be motor dependent, but rather this treatment had an effect on the structure of the surrounding chromatin.Citation21 Molecular beacons were used to tag a specific mRNA and similar conclusions were drawn.Citation54 In all of the studies, diffusion coefficients were extracted. These numbers can be translated to the time it would take an mRNP to find its way from the gene to the NPC. Diffusion coefficients of ∼0.04 µm2/sec as measured in several of the later studiesCitation21,Citation54,Citation55 were modeled and showed that mRNP travel would take on the range of 6 minutes.Citation56
To examine the time-frame of nuclear egress of mRNA in a comparative manner we generated a series of genes under inducible transcriptional control. The smallest mRNA was 1.7 kb in length whereas the longest one was 14 kb.Citation57 Some of the genes contained introns while others did not. The 10-fold difference in mRNA size showed significant differences in diffusion coefficients, which translated into calculated nucleoplasmic travel times in the range of 5 to 40 minutes. These calculated numbers were found comparable to the actual times of detection of mRNPs appearing in the cytoplasm, with measurements beginning from the initial point of transcription. Altogether, this analysis demonstrated that the time-frame required for the nucleoplasmic diffusion of mRNPs to the NPC was dependent on mRNA size, and occurred in the range of minutes. There was no difference in diffusion coefficients between the exact same transcript either with or without an intron. This would suggest that although the buildup of the mRNP mass can involve different protein components—since spliced mRNPs will contain the many components of the EJC whereas ones lacking an intron will not; nonetheless, the final structural arrangement of the mRNP will be similar, thus not affecting the diffusion properties of the mRNA particle.
Yet, a difference was seen in the efficiency of export between the intron-containing and intronless mRNAs. When the levels of both transcripts were high due to the inducible nature of the gene, the intronless transcript began to accumulate in the nucleus while the mRNA containing an intron continued to traffic to the cytoplasm. Indeed, the enhancing effect of splicing on gene expression has been known for many years, yet the exact effect on export has been debatable.Citation58–Citation60 Our data point to a direct effect of splicing on export. These experiments show that both types of transcripts can exit the nucleus when levels of transcription are coordinated with the rates of export.Citation41,Citation57 However, when the levels of the transcripts lacking an intron increase, the export system does not efficiently transport these transcripts out of the nucleus. This might be related to a limiting amount of an export protein that is required specifically for intronless mRNAs, whereas spliced mRNAs would already contain these proteins, which are recruited by the EJC proteins through the act of splicing. Indeed, when the inducible gene reduces its output, the intronless transcripts slowly empty out of the nucleus. Altogether, this analysis shows that splicing has an enhancing effect on mRNA export but does not influence the kinetics of nucleoplasmic transport.
The fact that the cell manages to sustain the gene expression response with a diffusional mechanism of nuclear mRNA transport is an intriguing observation. This would mean that the time-range of minutes for nucleoplasmic mRNA translocation is evolutionarily conserved in higher eukaryotes that have similar sized nuclei, and similar numbers of evolutionarily conserved NPCs. Yet, one could ponder the lack of directionality in a process that inherently harbors a defined direction, namely the mRNP must leave the transcription site to end up in the cytoplasm. To shed light on this issue we examined the pathways of mRNA with respect to the chromatin domain. We imaged live cells with labeled chromatin and tagged mRNPs. We could detect the “footsteps” of the mRNPs frame by frame in the movie sequences by using an image analysis method termed “maximum projection”. This simply means that the most pronounced footsteps of mRNPs in all movie frames were collected into one frame (). This analysis demonstrated that: (a) mRNPs are moving most of the time; (b) very few mRNPs are static, and therefore there are no obvious “rest-stops” for mRNPs; (c) mRNPs do not access nucleoli, the factories of rRNA synthesis; and (d) the mRNPs travel in pathways that snake in between dense chromatin regions (several mRNPs utilize the same path again and again).Citation57
mRNP tracks are consistent with observations that have suggested a reticular network of nucleoplasmic pathways that end up at the NPC.Citation50,Citation52,Citation61,Citation62 This was also suggested in the seminal hypothesis of “gene-gating”.Citation63 Indeed, electron microscopy has shown that the path leading up to NPCs consists of a space which could be termed inter-chromatin.Citation64 These tracks might correspond with the theory of the chromosome territory-interchromatin compartment (CT-IC) put forward by Thomas Cremer.Citation65 This group has shown that hyper-osmolar treatment of cells condenses the chromatin domain and exposes the actual large dimensions of the inter-chromatin domain.Citation66 We used this technique to show that mRNPs that were stuck in large inter-chromatin spaces exhibited movement that was more constrained. We therefore propose that the reticular track system in which mRNPs move, reduces the dimensionality of the movement decisions that the mRNP makes on the way to the pore. A channeled pathway that has only an “in” and “out” is a simple task compared to a large room with many doors (many decisions). Thereby the cell has harnessed channeled diffusion as a simple means by with to “direct” mRNPs from gene to pore.
Kinetics at the Pore
Live-cell studies of cargo import through the pore have reached single molecule resolution.Citation67–Citation72 However, studies of single mRNA export have lingered behind due to lack of appropriate cell systems.Citation73 The inducible gene system we generated transcribed mRNAs of large sizes (8 kb, 14 kb), and were designed such that the large tagged mRNPs could be identified when interacting with the nuclear pore and ultimately during export.Citation57 Indeed, large mRNPs could be detected as they reached the NPC and were transported through. Thinking of the channeled nucleoplasmic paths that ended at the channel of the NPC, it was of interest to compare the kinetics of the two processes. Diffusion through the channels was 15-fold slower than the movement through the pore. Since our measurements did not provide rapid temporal resolution we estimated the rate of mRNA passage through the pore to be in the 500 msec range or less, which correlated with previously measured kinetics of cargo translocation through the pore.Citation72,Citation74 This rapid egress would suggest a mechanism of facilitated mRNP exit through the pore compared to relatively slow diffusion in the nucleoplasm.Citation75
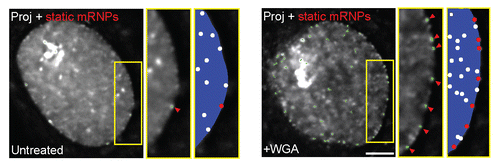
Studying the dynamics of mRNP anchorage and passage through the pore led to some interesting insights. Queues of mRNPs were not detected at the nuclear envelope. mRNPs mostly bumped back and forth on the envelope, and when attachment was successful they went directly through. Inhibition of export by use of the long established technique of wheat germ agglutinin (WGA) inhibition produced a unique picture in which many mRNPs were found anchored to the nuclear periphery ( and ). Most likely this anchoring occurs at the NPC since a recent study in C. tentans has shown by EM that mRNPs in WGA-treated cells were anchored to the nuclear basket.Citation76 This would mechanistically mean that mRNP anchoring is independent of mRNP export.
The mRNPs appearing in the cytoplasm had an open structure at first, which later returned to the characteristic round-shaped mRNP. This was indicative of mRNP restructuring during export, and would suggest that mRNPs change form in order to thread through the NPC. Such restructuring during export has been demonstrated for the large Balbiani ring mRNPs as seen by EM images.Citation13 Indeed, mRNP restructuring would be expected during export since the inner dimensions of the NPC are probably smaller than the diameter of an mRNP. Change of structure would also agree with the remodeling of mRNP components prior to or during export.Citation77
New insights on the gene expression pathway unfold before us as the capabilities of live-cell imaging expand. It is now possible to visualize the various elements of the pathway from genes, through mRNAs to proteins. Future studies will provide a better picture of the interactions of different processing and regulatory machineries within the path of expression. Development of high-resolution and rapid imaging systems will allow higher resolution of these measurements and will enable studies at the levels of single genes and single molecules. These should provide exciting new revelations on the fascinating machineries involved in the nucleo-cytoplasmic passage of an mRNA.
Abbreviations
| NPC | = | nuclear pore complex |
| UTR | = | untranslated region |
Figures and Tables
Figure 1 Following mRNPs in living cells. (A) Scheme of the MS2 system used for tagging mRNAs in living cells. The transcription of a gene of interest is driven by a promoter. The 3′UTR of the gene contains a series of MS2 sequence repeats, which fold into stem-loop structures in the transcribed mRNA. These are bound by YFP-MS2-CP proteins to yield the mRNP fluorescent. (B) Scheme of the travels of mRNPs from the site of transcription to the cytoplasm. Top-RNA polymerase II (green) recruited to a gene of interest generates mRNPs (red) that travel in between dense chromosomal territories (blue) and nucleoli (cyan) to the NPC. mRNPs are depicted as either unfolded or folded during transcription and translocation through the NPC. mRNPs diffuse through the nucleoplasm and can show corralled movement probably due to the hindering by the chromatin environment. Bottom-mRNA export is blocked in WGA (yellow) treated cells, and mRNPs remain anchored at the NPC.
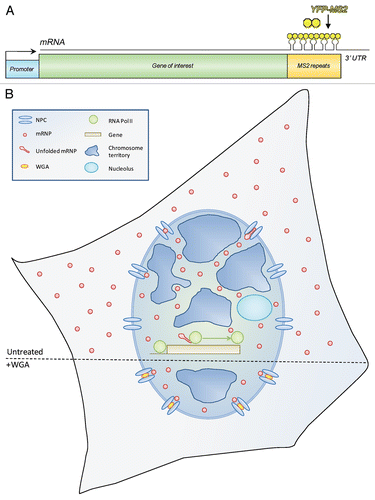
Figure 2 Identifying nucleoplasmic mRNP tracks using maximum time projections. (A) Demonstration of the application of the maximum time projection function to time-lapse movies. Top-scheme showing the pathway of a single spot over time; bottom—single frames from a movie of YFP-MS2 labeled mRNPs. The image on the right is the maximum time projection of all the single frames. (B) Maximum time projection of mRNPs imaged for two different time periods, in the same cell shown in (A): red-maximum time projection (M t proj.) of the first 30 movie frames (captured every second); green-maximum time projection from the last 30 seconds of the movies. Hoechst DNA staining and co-localized tracks from the different times are shown. White dashed line represents one of the nuclear tracks. Right-enlargement of the boxed area. Scale bars, 5 µm.
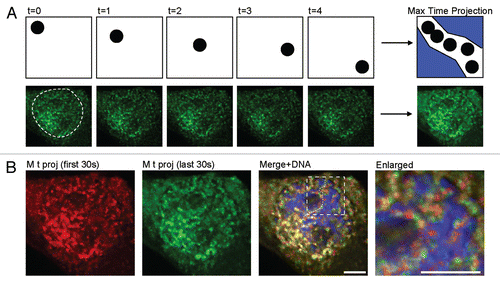
Figure 3 Blocking mRNP export by WGA treatment. Treatment with WGA leads to an increase in peripherally stalled mRNPs. Average time projection shows the stalled mRNPs and their tracks (green, by single particle tracking), also seen in an enlarged area (yellow box, middle). An increase in membrane stalled mRNPs is seen (left - same cell before treatment) after (right) treatment with WGA. Green tracks show all static mRNPs. The rest of the mRNPs continue to move in the nucleoplasm. Schemes on right show the dynamic mRNPs (white dots) and the static mRNPs (red dots). Scale bar, 5 µm. Adapted from reference Citation57.
Acknowledgements
Y.S.T. thanks the funding agencies that supported these studies: Israel Science Foundation (grant 250/06), the Israel Science Foundation Bikura grant, United States-Israel Binational Science Foundation, Israel Cancer Research Fund, Israel Cancer Association, the Israel Ministries of Health and Science, and the Alon Fellowship. Y.S.T. is the Jane Stern Lebell Family Fellow in Life Sciences at BIU.
Extra View on: Mor A, Suliman S, Ben-Yishay R, Yunger S, Brody Y, Shav-Tal Y. Dynamics of single mRNP nucleo-cytoplasmic transport through the nuclear pore in living cells. Nat Cell Biol 2010; 12:543 - 552; PMID: 20453848; http://dx.doi.org/10.1038/ncb2056
References
- de Almeida SF, Carmo-Fonseca M. The CTD role in cotranscriptional RNA processing and surveillance. FEBS Lett 2008; 582:1971 - 1976
- Munoz MJ, de la Mata M, Kornblihtt AR. The carboxy terminal domain of RNA polymerase II and alternative splicing. Trends Biochem Sci 2010; 35:497 - 504
- Neugebauer KM. On the importance of being co-transcriptional. J Cell Sci 2002; 115:3865 - 3871
- Perales R, Bentley D. “Cotranscriptionality”: the transcription elongation complex as a nexus for nuclear transactions. Mol Cell 2009; 36:178 - 191
- Izaurralde E, Lewis J, McGuigan C, Jankowska M, Darzynkiewicz E, Mattaj IW. A nuclear cap binding protein complex involved in pre-mRNA splicing. Cell 1994; 78:657 - 668
- Wahl MC, Will CL, Luhrmann R. The spliceosome: design principles of a dynamic RNP machine. Cell 2009; 136:701 - 718
- Le Hir H, Izaurralde E, Maquat LE, Moore MJ. The spliceosome deposits multiple proteins 20–24 nucleotides upstream of mRNA exon-exon junctions. EMBO J 2000; 19:6860 - 6869
- Le Hir H, Nott A, Moore MJ. How introns influence and enhance eukaryotic gene expression. Trends Biochem Sci 2003; 28:215 - 220
- Maniatis T, Reed R. An extensive network of coupling among gene expression machines. Nature 2002; 416:499 - 506
- Dreyfuss G, Kim VN, Kataoka N. Messenger-RNA-binding proteins and the messages they carry. Nat Rev Mol Cell Biol 2002; 3:195 - 205
- Rosonina E, Kaneko S, Manley JL. Terminating the transcript: breaking up is hard to do. Genes Dev 2006; 20:1050 - 1056
- Reed R, Magni K. A new view of mRNA export: separating the wheat from the chaff. Nat Cell Biol 2001; 3:201 - 204
- Mehlin H, Daneholt B, Skoglund U. Translocation of a specific premessenger ribonucleoprotein particle through the nuclear pore studied with electron microscope tomography. Cell 1992; 69:605 - 613
- Cheng H, Dufu K, Lee CS, Hsu JL, Dias A, Reed R. Human mRNA export machinery recruited to the 5′ end of mRNA. Cell 2006; 127:1389 - 1400
- Isken O, Maquat LE. Quality control of eukaryotic mRNA: safeguarding cells from abnormal mRNA function. Genes Dev 2007; 21:1833 - 1856
- Behm-Ansmant I, Izaurralde E. Quality control of gene expression: a stepwise assembly pathway for the surveillance complex that triggers nonsense-mediated mRNA decay. Genes Dev 2006; 20:391 - 398
- Shav-Tal Y, Singer RH, Darzacq X. Imaging gene expression in single living cells. Nat Rev Mol Cell Biol 2004; 5:855 - 861
- Hager GL, McNally JG, Misteli T. Transcription dynamics. Mol Cell 2009; 35:741 - 753
- Darzacq X, Yao J, Larson DR, Causse SZ, Bosanac L, de Turris V, et al. Imaging transcription in living cells. Annu Rev Biophys 2009; 38:173 - 196
- Fusco D, Accornero N, Lavoie B, Shenoy SM, Blanchard JM, Singer RH, et al. Single mRNA molecules demonstrate probabilistic movement in living mammalian cells. Curr Biol 2003; 13:161 - 167
- Shav-Tal Y, Darzacq X, Shenoy SM, Fusco D, Janicki SM, Spector DL, et al. Dynamics of single mRNPs in nuclei of living cells. Science 2004; 304:1797 - 1800
- Bertrand E, Chartrand P, Schaefer M, Shenoy SM, Singer RH, Long RM. Localization of ASH1 mRNA particles in living yeast. Mol Cell 1998; 2:437 - 445
- Golding I, Cox EC. RNA dynamics in live Escherichia coli cells. Proc Natl Acad Sci USA 2004; 101:11310 - 11315
- Golding I, Paulsson J, Zawilski SM, Cox EC. Real-time kinetics of gene activity in individual bacteria. Cell 2005; 123:1025 - 1036
- Forrest KM, Gavis ER. Live imaging of endogenous RNA reveals a diffusion and entrapment mechanism for nanos mRNA localization in Drosophila. Curr Biol 2003; 13:1159 - 1168
- Becalska AN, Gavis ER. Lighting up mRNA localization in Drosophila oogenesis. Development 2009; 136:2493 - 2503
- Chubb JR, Trcek T, Shenoy SM, Singer RH. Transcriptional pulsing of a developmental gene. Curr Biol 2006; 16:1018 - 1025
- Stevense M, Muramoto T, Muller I, Chubb JR. Digital nature of the immediate-early transcriptional response. Development 2010; 137:579 - 584
- Darzacq X, Shav-Tal Y, de Turris V, Brody Y, Shenoy SM, Phair RD, et al. In vivo dynamics of RNA polymerase II transcription. Nat Struct Mol Biol 2007; 14:796 - 806
- Boireau S, Maiuri P, Basyuk E, de la Mata M, Knezevich A, Pradet-Balade B, et al. The transcriptional cycle of HIV-1 in real-time and live cells. J Cell Biol 2007; 179:291 - 304
- Tsukamoto T, Hashiguchi N, Janicki SM, Tumbar T, Belmont AS, Spector DL. Visualization of gene activity in living cells. Nat Cell Biol 2000; 2:871 - 878
- Tumbar T, Sudlow G, Belmont AS. Large-scale chromatin unfolding and remodeling induced by VP16 acidic activation domain. J Cell Biol 1999; 145:1341 - 1354
- Janicki SM, Tsukamoto T, Salghetti SE, Tansey WP, Sachidanandam R, Prasanth KV, et al. From silencing to gene expression; real-time analysis in single cells. Cell 2004; 116:683 - 698
- Lamond AI, Swedlow JR. RNA polymerase II transcription in living color. Nat Struct Mol Biol 2007; 14:788 - 790
- Femino AM, Fay FS, Fogarty K, Singer RH. Visualization of single RNA transcripts in situ. Science 1998; 280:585 - 590
- O'Brien T, Lis JT. Rapid changes in Drosophila transcription after an instantaneous heat shock. Mol Cell Biol 1993; 13:3456 - 3463
- Tennyson CN, Klamut HJ, Worton RG. The human dystrophin gene requires 16 hours to be transcribed and is cotranscriptionally spliced. Nat Genet 1995; 9:184 - 190
- Ucker DS, Yamamoto KR. Early events in the stimulation of mammary tumor virus RNA synthesis by glucocorticoids. Novel assays of transcription rates. J Biol Chem 1984; 259:7416 - 7420
- Yunger S, Rosenfeld L, Garini Y, Shav-Tal Y. Single-allele analysis of transcription kinetics in living mammalian cells. Nat Methods 2010; 7:631 - 633
- Dundr M, Hoffmann-Rohrer U, Hu Q, Grummt I, Rothblum LI, Phair RD, et al. A kinetic framework for a mammalian RNA polymerase in vivo. Science 2002; 298:1623 - 1626
- Ben-Ari Y, Brody Y, Kinor N, Mor A, Tsukamoto T, Spector DL, et al. The life of an mRNA in space and time. J Cell Sci 2010; 123:1761 - 1774
- Sehgal PB, Derman E, Molloy GR, Tamm I, Darnell JE. 5,6-Dichloro-1-Beta-D-ribofuranosylbenzimidazole inhibits initiation of nuclear heterogeneous RNA chains in HeLa cells. Science 1976; 194:431 - 433
- Papantonis A, Larkin JD, Wada Y, Ohta Y, Ihara S, Kodama T, et al. Active RNA polymerases: mobile or immobile molecular machines?. PLoS Biol 2010; 8:1000419
- Wada Y, Ohta Y, Xu M, Tsutsumi S, Minami T, Inoue K, et al. A wave of nascent transcription on activated human genes. Proc Natl Acad Sci USA 2009; 106:18357 - 18361
- Singh J, Padgett RA. Rates of in situ transcription and splicing in large human genes. Nat Struct Mol Biol 2009; 16:1128 - 1133
- de la Mata M, Alonso CR, Kadener S, Fededa JP, Blaustein M, Pelisch F, et al. A slow RNA polymerase II affects alternative splicing in vivo. Mol Cell 2003; 12:525 - 532
- Munoz MJ, Perez Santangelo MS, Paronetto MP, de la Mata M, Pelisch F, Boireau S, et al. DNA damage regulates alternative splicing through inhibition of RNA polymerase II elongation. Cell 2009; 137:708 - 720
- Shav-Tal Y, Gruenbaum Y. Single-molecule dynamics of nuclear mRNA. F1000 Biology Reports 2009; 1:29 - 32
- Politz JC, Browne ES, Wolf DE, Pederson T. Intranuclear diffusion and hybridization state of oligonucleotides measured by fluorescence correlation spectroscopy in living cells. Proc Natl Acad Sci USA 1998; 95:6043 - 6048
- Politz JC, Tuft RA, Pederson T, Singer RH. Movement of nuclear poly(A) RNA throughout the interchromatin space in living cells. Curr Biol 1999; 9:285 - 291
- Singh OP, Bjorkroth B, Masich S, Wieslander L, Daneholt B. The intranuclear movement of Balbiani ring premessenger ribonucleoprotein particles. Exp Cell Res 1999; 251:135 - 146
- Zachar Z, Kramer J, Mims IP, Bingham PM. Evidence for channeled diffusion of pre-mRNAs during nuclear RNA transport in metazoans. J Cell Biol 1993; 121:729 - 742
- Calapez A, Pereira HM, Calado A, Braga J, Rino J, Carvalho C, et al. The intranuclear mobility of messenger RNA binding proteins is ATP dependent and temperature sensitive. J Cell Biol 2002; 159:795 - 805
- Vargas DY, Raj A, Marras SA, Kramer FR, Tyagi S. Mechanism of mRNA transport in the nucleus. Proc Natl Acad Sci USA 2005; 102:17008 - 17013
- Molenaar C, Abdulle A, Gena A, Tanke HJ, Dirks RW. Poly(A)+ RNAs roam the cell nucleus and pass through speckle domains in transcriptionally active and inactive cells. J Cell Biol 2004; 165:191 - 202
- Braga J, Rino J, Carmo-Fonseca M. Photobleaching microscopy reveals the dynamics of mRNA-binding proteins inside live cell nuclei. Prog Mol Subcell Biol 2004; 35:119 - 134
- Mor A, Suliman S, Ben-Yishay R, Yunger S, Brody Y, Shav-Tal Y. Dynamics of single mRNP nucleocytoplasmic transport and export through the nuclear pore in living cells. Nat Cell Biol 2010; 12:543 - 552
- Luo MJ, Reed R. Splicing is required for rapid and efficient mRNA export in metazoans. Proc Natl Acad Sci USA 1999; 96:14937 - 14942
- Valencia P, Dias AP, Reed R. Splicing promotes rapid and efficient mRNA export in mammalian cells. Proc Natl Acad Sci USA 2008; 105:3386 - 3391
- Lu S, Cullen BR. Analysis of the stimulatory effect of splicing on mRNA production and utilization in mammalian cells. RNA 2003; 9:618 - 630
- Lawrence JB, Singer RH, Marselle LM. Highly localized tracks of specific transcripts within interphase nuclei visualized by in situ hybridization. Cell 1989; 57:493 - 502
- Reichenzeller M, Burzlaff A, Lichter P, Herrmann H. In vivo observation of a nuclear channel-like system: evidence for a distinct interchromosomal domain compartment in interphase cells. J Struct Biol 2000; 129:175 - 185
- Blobel G. Gene gating: a hypothesis. Proc Natl Acad Sci USA 1985; 82:8527 - 8529
- Fakan S. The functional architecture of the nucleus as analysed by ultrastructural cytochemistry. Histochem Cell Biol 2004; 122:83 - 93
- Cremer T, Cremer M. Chromosome territories. Cold Spring Harb Perspect Biol 2010; 2:3889
- Albiez H, Cremer M, Tiberi C, Vecchio L, Schermelleh L, Dittrich S, et al. Chromatin domains and the interchromatin compartment form structurally defined and functionally interacting nuclear networks. Chromosome Res 2006; 14:707 - 733
- Yang W, Gelles J, Musser SM. Imaging of single-molecule translocation through nuclear pore complexes. Proc Natl Acad Sci USA 2004; 101:12887 - 12892
- Yang W, Musser SM. Nuclear import time and transport efficiency depend on importin beta concentration. J Cell Biol 2006; 174:951 - 961
- Dange T, Grunwald D, Grunwald A, Peters R, Kubitscheck U. Autonomy and robustness of translocation through the nuclear pore complex: a single-molecule study. J Cell Biol 2008; 183:77 - 86
- Kubitscheck U, Grunwald D, Hoekstra A, Rohleder D, Kues T, Siebrasse JP, et al. Nuclear transport of single molecules: dwell times at the nuclear pore complex. J Cell Biol 2005; 168:233 - 243
- Siebrasse JP, Peters R. Rapid translocation of NTF2 through the nuclear pore of isolated nuclei and nuclear envelopes. EMBO Rep 2002; 3:887 - 892
- Ribbeck K, Gorlich D. Kinetic analysis of translocation through nuclear pore complexes. EMBO J 2001; 20:1320 - 1330
- Mor A, Ben-Yishay R, Shav-Tal Y. Dynamics and kinetics of nucleo-cytoplasmic mRNA export. Wiley Interdisciplinary Reviews—RNA 2010; In press
- Carmo-Fonseca M. Follow that messenger: live-imaging a journey out of the nucleus. Dev Cell 2010; 18:880 - 882
- Noble KN, Wente SR. Nuclear mRNA on the move. Nat Cell Biol 2010; 12:525 - 527
- Kylberg K, Bjork P, Fomproix N, Ivarsson B, Wieslander L, Daneholt B. Exclusion of mRNPs and ribosomal particles from a thin zone beneath the nuclear envelope revealed upon inhibition of transport. Exp Cell Res 2010; 316:1028 - 1038
- Carmody SR, Wente SR. mRNA nuclear export at a glance. J Cell Sci 2009; 122:1933 - 1937