Abstract
Lamins are the major components of the nuclear lamina, a filamentous layer found at the interphase between chromatin and the inner nuclear membrane. The lamina supports the nuclear envelope and provides anchorage sites for chromatin. Lamins and their associated proteins are required for most nuclear activities, mitosis, and for linking the nucleoskeleton to the network of cytoskeletal filaments. Mutations in lamins and their associated proteins give rise to a wide range of diseases, collectively called laminopathies. This review focuses on the evolution of the lamin protein family. Evolution from basal metazoans to man will be described on the basis of protein sequence comparisons and analyses of their gene structure. Lamins are the founding members of the family of intermediate filament proteins. How genes encoding cytoplasmic IF proteins could have arisen from the archetypal lamin gene progenitor, can be inferred from a comparison of the respective gene structures. The lamin/IF protein family seems to be restricted to the metazoans. In general, invertebrate genomes harbor only a single lamin gene encoding a B-type lamin. The archetypal lamin gene structure found in basal metazoans is conserved up to the vertebrate lineage. The completely different structure of lamin genes in Caenorhabditis and Drosophila are exceptions rather than the rule within their systematic groups. However, variation in the length of the coiled-coil forming central domain might be more common than previously anticipated. The increase in the number of lamin genes in vertebrates can be explained by two rounds of genome duplication. The origin of lamin A by exon shuffling might explain the processing of prelamin A to the mature non-isoprenylated form of lamin A. By alternative splicing the number of vertebrate lamin proteins has increased even further. Lamin C, an alternative splice form of the LMNA gene, is restricted to mammals. Amphibians and mammals express germline-specific lamins that differ in their protein structure from that of somatic lamins. Evidence is provided that there exist lamin-like proteins outside the metazoan lineage.
Introduction
In eukaryotic cells the nucleoplasmic and the cytoplasmic compartments are separated by the nuclear envelope. The nuclear envelope consists of two lipid bilayers, the inner and outer nuclear membranes. The two nuclear membranes are fused at sites where nuclear pore complexes are embedded. The nucleoplasmic side of the inner nuclear membrane of metazoans is closely associated with a fibrous protein network, the nuclear lamina. A “complex supporting layer of fine filaments closely applied to the inner aspect of the nuclear envelope”Citation1 has been described in the fifties of the last century.Citation2 Notably, the first observations of such a filamentous layer were not found in metazoans but in two unicellular eukaryotesCitation2-Citation4 followed by reports on nerve cells of an invertebrateCitation5-Citation7 and from different vertebrates including humans.Citation1,Citation8,Citation9 These early electron microscopy reports are noteworthy because they show how prominent (30–200 nm thick) this layer might be in certain cells.Citation1-Citation9 The isolation of a pore complex-lamina fraction from rat liver nucleiCitation10,Citation11 and the immunohistochemical characterization of lamin proteinsCitation12 established that lamins are the main components of the lamina. Finally, ultrastructural analysis of amphibian oocyte nuclei and sequencing data from human lamins demonstrated that lamins are members of the multigene family of intermediate filament (IF) proteins.Citation13-Citation15 Based on their sequence characteristics and their assembly properties they are classified as type V IF proteins.Citation16
Lamins have the tripartite domain organization common to all IF proteins.Citation16 A central α-helical rod domain, able to form coiled-coils, is flanked by a short N-terminal and a larger C-terminal domain. The rod domain is characterized by a heptad substructure over the majority of its length, in which every first and fourth amino acid position in a sequence of seven amino acid residues is occupied by a hydrophobic residue. This allows for the formation of the coiled-coil structure between two molecules giving rise to lamin dimers.Citation17 The rod domain is divided into two coiled-coil forming segments, coil 1 and coil 2 which are connected by linker L12. Coils 1 and 2 contain the linker sequences L1 and L2 which do not exhibit α-helical organization on their own; however, it has been proposed that in the dimer state these sequences form bundles of paired α-helices.Citation18 The length of the lamin rod domain is conserved in most organisms. Moreover, the end segments on either sides of the rod are highly conserved. These so-called IF consensus motifs are present in every IF protein in nearly identical form.Citation19-Citation21 Head and tail domains of lamins are predicted to be very flexible without exhibiting pronounced secondary structures except for a globular domain within the tail, which resembles the immunoglobulin (Ig) structure. This Ig-domain consists of about 110 residues folded into a β-sandwich of nine β-strands.Citation22,Citation23 The core of this domain is formed by hydrophobic residues, while most charged residues occur at the surface of the domain, allowing interactions with lamins, other proteins, and DNA.Citation16
The designation of lamins as lamin A, B, and C refers back to the work with rat liver lamins and simply reflects their increasing gel electrophoretic mobility.Citation24 Later, lamins were classified as A-type or B-type lamins according to their expression patterns, behavior during mitosis and biochemical characteristics.Citation25 While this classification fits in for most of the cases, from an evolutionary point of view it is not justified for all lamins, as will be described in this review.
Lamin assembly differs from that of cytoplasmic IFs in certain aspects. The first assembly step involves formation of lamin dimers, which is followed by formation of polar head to tail polymers. It is suggested that the head to tail interaction is mediated by the IF consensus motifs.Citation18 Next, antiparallel lateral assembly of two head to tail polymers form a non-polar protofilament and three to four protofilaments can then assemble into 10 nm filaments.Citation26 The exact mechanism of assembly is not fully understood, however, transmission and scanning electron microscopic analyses have revealed that lamins form long filaments in vivo having a diameter of about 10 to 15 nm.Citation13,Citation27
In addition to the general IF features, three sequence motifs are characteristic for lamins: A basic nuclear localization signal (NLS) within the C-terminus, a CaaX motif at the very C-terminus, and a cyclin-dependent kinase 1 (CDK1) phosphorylation site in front of the rod domain. While the NLS avails import into the nucleus, the CaaX motif serves as a target for a series of posttranslational modifications, which increase the hydrophobicity of the lamins and thereby enable interactions with the inner nuclear membrane. The motif contains a cysteine residue (C) followed by two aliphatic residues (aa). The cysteine residue serves as an acceptor site for isoprenylation. The fourth residue (X) determines the type of isoprene moiety, which is added to the cysteine. In case of lamins, a farnesyl group is transferred to the cysteine, forming a thioether bond. After addition of the isoprene moiety the last three amino acid residues (aaX) are cleaved off by either of the two endoproteases Rce1 or ZMPSTE21, followed by carboxyl-methylation of the newly formed C-terminus.Citation28 All lamins that carry a CaaX motif undergo this modification, but it must be noted that A-type lamins, which exhibit an additional ZMPSTE24 protease cleavage site in their tail region, loose the lipid modification upon processing by this protease.Citation29 Therefore, B-type lamins, which do not undergo this second proteolytic processing step, remain permanently isoprenylated while fully processed A-type lamins are recruited to the nuclear envelope via interactions with lamins and/or lamin-associated proteins.Citation30,Citation31
The highly conserved CDK1 recognition site in front of the rod domain, is of crucial importance for the reversible disassembly of the nuclear laminaCitation24 in the course of nuclear envelope breakdown during M-phase of the cell cycle. Disassembly of lamin filaments is triggered by CDK1 phosphorylation at this siteCitation32-Citation34 and reassembly starts upon dephosphorylation.Citation35
The nuclear lamina is an essential component of metazoan cells.Citation36,Citation37 It forms the main component of the nuclear skeleton and contributes to mechanical stability of the nuclear envelope and is involved in anchoring nuclear pore complexes.Citation38-Citation40 Lamins interact with a wealth of proteins to fulfill a similar wealth of cellular functions.Citation25 Lamins take part in regulation of chromatin organization,Citation41 DNA-replication and RNA-transcription,Citation42 nuclear size determinationCitation43 and cell cycle regulation.Citation44,Citation45 Furthermore, through interaction with SUN-domain proteins lamins interact with nesprins, which bind microtubules, actin- and cytoplasmic IFs, linking the cytoskeleton to the nucleoskeleton.Citation46-Citation48 The importance of lamin function is highlighted by mutations in genes encoding lamins, which cause a wide range of heritable human diseases, collectively termed laminopathies. Mutations in the human LMNA gene affect muscle, adipose, bone, nerve and skin cells. One group of laminopathies which exhibit particularly severe phenotypes comprises progeroid syndromes such as Hutchinson-Gilford progeria syndrome, atypical Werner syndrome and mandibuloacral dysplasia.Citation25,Citation48
Structure, assembly as well as the multitude of cell biological aspects of lamins and particularly their role in laminopathies is currently been described in a growing number of excellent reviews.Citation25,Citation48-Citation57 In this review we will focus on the evolution of the lamin protein family. Here we review relevant literature and present new data obtained by sequence data bank searches.
An Interjection at the Beginning
A comparison of intron patterns might seem to be very dry matter for those who devote all their attention to the biological functions of lamins. But, with the evolution of species, nature has done experiments for us, which we probably would neither plan nor for which grant agencies would give us the money, but they can teach us a lot.
Lamins are the Founding Members of the IF Multigene Family
With the complete cDNA sequence of human lamin A the relationship between nuclear lamins and cytoplasmic IF proteins was discovered.Citation14,Citation15 It was speculated, that lamins are the founding members of the IF multigene family.Citation58 First direct support for this speculation came from protein sequence comparisons, which showed that the coiled-coil rod domains of lamins and invertebrate IF proteins have the same length, while those of vertebrate IF proteins are shorter by six heptads. Moreover, within their tail domains, lamins show significant sequence similarity to invertebrate but not to vertebrate IF proteins. Cytoplasmic IF proteins of invertebrates are therefore closer to nuclear lamins than to the cytoplasmic IFs of vertebrates.Citation59,Citation60 Analysis of the exon/intron pattern of a vertebrate nuclear lamin gene, the LIII gene of Xenopus laevis, and an invertebrate gene of the gastropod Helix aspersa encoding a cytoplasmic IF protein has revealed a remarkable similarity in the structural organization of these genes.Citation61,Citation62 Eight out of ten introns present in both genes are located at homologous positions. This striking similarity substantiated the common ancestry of lamins and IF proteins. The few differences in intron/exon patterns also instantly suggested how the archetype IF protein gene might have arisen from a lamin-like progenitor (). Deletion of two signal sequences found in lamins but not in IF proteins convert a nuclear lamin into a cytoplasmic IF protein. These two signals are the NLS and the C-terminal CaaX motif. During evolution removal of the NLS could have been achieved by creation of splice recognition sites flanking the NLS. This would result in the removal of this signal during RNA maturation and consequently, in the generation of a new intron in the gene. Sequence alignments of lamins and invertebrate IF proteins necessitate the introduction of a gap in the IF sequence, which coincides with the position of the NLS in lamins.Citation59 The Helix IF gene has an intron in this region, which has no counterpart in the lamin gene. The last intron, which delineates a short exon and encodes the CaaX motif in lamin genes, is absent in the IF gene. Introduction of a stop codon in the preceding exon of the lamin gene would delete the CaaX encoding exon and would shorten the protein chain of the resulting IF protein. The elimination of the two signal sequences freed the lamin-like archetype IF protein from nuclear compartmentalization and membrane interactions and provided the possibility to form cytoplasmic IFs.
Figure 1. A model of IF evolution from an ancestral lamin progenitor. Intron positions (triangles, numbered with Roman numerals) are shown with respect to protein structure. Large boxes represent coiled-coil segments. The oval represents the lamin Ig-domain. Archetypal intron positions are labeled in red, introns gained in the course of cytoplasmic IF evolution are in blue. Deletion of the region containing the NLS is indicated by gray lines, the resulting new intron is marked by a star. Invertebrate IF proteins lack the last CaaX-encoding exon. Coil 1b in vertebrate IF proteins is shorter by six heptads. The deletion of the six heptads can be explained by generation of a new splice acceptor site of intron I. The heptads, which become deleted, are marked by dashed lines in coil 1b of the invertebrate IF. The tail domains of vertebrate IFs do not show resemblance to their invertebrate progenitors, marked by a hatched line. NLS, nuclear localization signal; CaaX, isoprenylation motif; CDK1, CDK1 phosphorylation site.
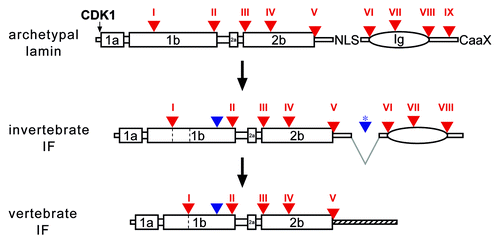
The evolution of vertebrate IFs was accompanied by the loss of six heptads in coil1b. The site of deletion coincides with the position of an archetypal intron. It is therefore likely that this deletion was caused by the formation of a new splice acceptor site.
The analysis of lamin genes outside the vertebrate group was initially focused on genetically tractable model organisms like Caenorhabditis elegansCitation63 and Drosophila melanogaster.Citation64 These two species, however, show a strong genome drift and their lamin genes have entirely different organization (see below).
The search for lamin genes in different taxonomic groups was initially done by mapping of exon/intron boundaries of cloned genomic fragments. The emergence of a constantly growing number of genome assemblies made sequence information available for a broad spectrum of species and data bank mining became the main source for this type of evolutionary studies. However, successful identification of new (lamin) genes critically depends on the quality of the genome data. Gaps in the assemblies may prevent discovery or unambiguous assignment of individual (lamin) genes. The gene encoding chicken lamin A, for example, is still not found in the published genome sequenceCitation65 although its cDNA sequence has been published two decades ago.Citation66 It would therefore be highly desirable to fill existing gaps in published genomes even if this would slow down the genome sequencing of further species.
The results presented here are mainly based on literature reports and, in addition, on our own databank searches. We have screened publicly available databases with lamin sequences from a large variety of species. In many cases, annotated gene sequences had to be manually re-edited or had to be extracted from scratch. When available, exon/intron boundaries were verified by comparing genomic sequences with the corresponding cDNA or EST sequences. Whenever these were not available, intron positions were inferred from protein sequence alignments and the presence of suitable splice donor and acceptor consensus motifs. This method provides reliable results for large parts of the lamin sequences, particularly for regions with high or moderately high sequence similarity. In some cases, e.g., in parts of the lamin tail that show little sequence conservation, this method might fail or produce ambiguous results. This applies particularly for the very short last exon, which encodes the CaaX sequence. Results solely based on genomic sequence information must therefore be considered with some caution.
From these analyses the following conclusions can be drawn: (1) Lamins seem to be restricted to metazoans, (2) invertebrate genomes possess a single lamin gene encoding a B-type lamin (for exceptions in certain insect groups see below), (3) the archetypal lamin gene structure, found in basic metazoans, is conserved in all vertebrate lamins, (4) within the protostome lineage gene structures differ most from that of the archetypal lamin gene, (5) the increased number of lamin genes in vertebrates can be explained by two rounds of whole genome duplications at the onset of vertebrate radiation,Citation67,Citation68 (6) lamin A is restricted to vertebrates. Its origin by exon shuffling might explain the processing of prelamin A to the mature non-isoprenylated lamin A. These points will be discussed in detail below.
The Metazoans
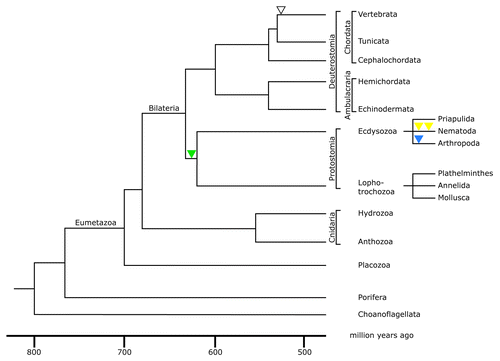
Metazoans constitute a monophyletic taxon, which includes all multicellular animals. The sister group of metazoans is formed by the choanoflagellates (). The most basal metazoans are the sponges, which form the phylum Porifera. Although sponges have different specialized cell types, these cells do not form tissues, as they are characteristic for all other metazoans. Sponges already differentiate oocytes and spermatozoa as generative cells. All other metazoans belong to the eumetazoan group. The most basal eumetazoans are the placozoans with the single genus Trichoplax. They are small disc-shaped animals, which consist of two epithelial layers separated by multinucleated fiber cells.Citation71 Phylogenetic analyses placed them as the basal eumetazoan lineage that diverged already before the cnidarians but after the divergence of sponges.Citation72,Citation73
Figure 2. Phylogenetic tree of the metazoan lineage. The tree was modified from reference Citation69. The time scale was taken from molecular clock results of reference Citation70. It is consistent with paleontological findings. Choanoflagellates were taken as the outgroup of metazoans for this phylogenetic tree. The green triangle indicates the acquisition of an intron restricted to the protostome lineage; yellow triangles indicate the acquisition of two introns restricted to nematodes; the blue triangle indicates acquisition of an intron restricted to arthropods; the open triangle represents the acquisition of an intron present in all vertebrate lamin genes.
The next branch in eumetazoan phylogeny is formed by the phylum Cnidaria comprising of the anthozoans and the hydrozoans. Fossil representatives are already known from the ediacaran era, which spans the period from 635 to 542 million years ago. Their sac-like body has a single opening, which is formed by two epithelial layers. Cnidarians developed a primitive nervous system, which lacks ganglia or other coordination centers.
All other metazoans show primary bilateral symmetry and are therefore grouped as Bilateria (). In some groups e.g., the echinoderms the bilateral symmetry has been lost secondarily. Bilateria are characterized by the presence of three germ layers, ectoderm, endoderm, and mesoderm. The latter develops during the process of gastrulation. The main lineages of the bilateria are formed by the protostomes and the deuterostomes (). The grouping of the two lineages is based on the fate of the primary body opening, the blastopore or urmund. In the protostomes the blastopore becomes the mouth opening while the anus is secondarily formed. In deuterostomes the opposite situation is found, here the blastopore becomes the anus. The first bilaterians evolved during the ediacaran era roughly 635–542 million years ago.Citation70 The protostomes are subdivided into lophotrochozoans (including plathelminths, annelids, and mollusks) and the ecdysozoans (including priapulids, nematodes, and arthropods). The deuterostomes comprise the echinoderms, hemichordates, and chordates. The latter are subdivided into the cephalochordates, tunicates, and vertebrates ().
Lamin Gene Organization is Conserved Between the Most Basal Metazoans and Mammals
After completion of the first sponge genome project,Citation74 lamin gene sequences are now available for at least one representative of each metazoan phylum. All invertebrate lamins show the same overall sequence organization typical for vertebrate lamins and resemble in size to the vertebrate B-type lamins. Comparison of the lamin genes of the basal metazoans, e.g., the poriferan Amphimedon queenslandica (a sponge), the placozoan Trichoplax adhaerens and the cnidarians Hydra magnipapillata, a hydrozoan, Nematostella vectensis, an anthozoan, and vertebrate lamins has been particularly informative. shows an alignment of these four basic metazoan lamins with human lamin B1. Lamin B1 was chosen as reference since it is thought to be the vertebrate ortholog of the invertebrate lamin.Citation77,Citation79 This assumption is based on microsynteny. The single lamin gene in Nematostella is flanked by the gene encoding the membrane-associated Ring finger protein 2/3. The same gene flanks the lamin B1 genes in Xenopus and man.Citation77 The sequence alignment of lamins from these distantly related species is nearly perfect (). It requires the introduction of a single gap within linker 12 of the Hydra rod domain to compensate for length heterogeneity of this region. The same applies to the conserved part of the tail, i.e., the Ig-domain, where small gaps have to be introduced. As seen for nearly all lamins, the remaining parts of the tail domain and the head domain vary in size between individual lamins.
Figure 3. The intron positions in lamin genes are conserved between the most basal metazoans and vertebrates. Alignment of predicted lamin protein sequences of the sponge Amphimedon queenslandica, the placozoan Trichoplax adhaerens, two cnidarians, Hydra magnipapillata, a hydrozoan, and Nematostella vectensis, an anthozoan, and human lamin B1 (Homo B1) was performed with the MultAlin softwareCitation75 using the Blosum62 matrix.Citation76 The sequence for the sponge was manually extracted from the Amphimedon genome project.Citation74 The Trichoplax, Hydra and Nematostella lamin sequences were taken from published data.Citation77 Trichoplax intron positions were re-inspected and a putative CaaX motif-encoding exon was identified and added to the Trichoplax sequence. The gene structure of human lamin B1 was taken from.Citation78 The position of all nine introns present in the lamin genes of the four basal metazoans is conserved in the human lamin. These intron positions are marked by white letters on red background and are numbered with roman numbers I to IX. Introns, which have no match in one of the other four lamin genes, are marked white on black. Where the exon/intron boundary is between two codons, the two adjacent amino acid residues are marked. In the other two cases the exon / intron boundary is between the second and third base of the codon of the marked amino acid residue. The short CaaX motif-encoding exon is heterogeneous in size. Consequently, intron positions of intron IX appear out of register in this alignment.
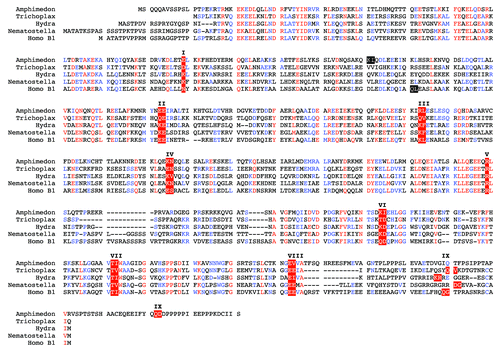
Particularly remarkable is the conservation of the intron pattern between the basal metazoans and the human lamin B1 gene, the prototype of the vertebrate lamins. The positions of nine introns, which are identical in the lamin genes of basal metazoans and in those of vertebrates, (I–IX in ) highlight the archetype of the lamin gene structure and hence that of all IF genes. Eight of these introns are also present in genes of cytoplasmic IFs of invertebratesCitation61 and five (introns I to V) are present in nearly all type I–III vertebrate IF genes.Citation61 It will become clear in the following description that the archetypal pattern of introns has been retained to very different extent in the different phyla of metazoans.
Lamins of the Protostome Lineage
Protostomes comprise two major groups, the lophotrochozoans and the ecdysozoans. The taxon Lophotrochozoa was discovered based on small-subunit rRNA sequence comparison.Citation80 It includes the flatworms (plathelminths), annelids and mollusks (). The ecdysozoan group covers the priapulids, nematodes, and arthropods. The ecdysozoan group houses two model organisms widely used in classical and molecular genetics and developmental biology, i.e., Caenorhabditis elegans and Drosophila melanogaster. Drosophila belongs to the phylum Arthropoda while Caenorhabditis is grouped to the more basal group of nematodes. The gene structures of lamin Dmo of DrosophilaCitation64 and the Ce-lamin of CaenorhabditisCitation63 were the first to be reported for invertebrates.
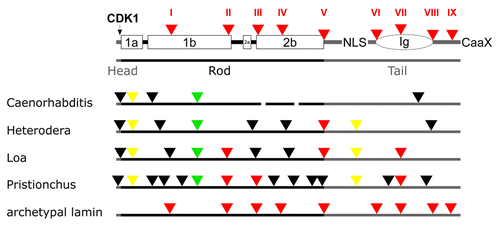
Noteworthy for protostome lamin genes is the presence of an intron in coil 1b, which is present in three of the lophotrochozoans and several ecdysozoans, i.e., all nematodes and several arthropods (marked green in , and ; Figs. S1–S4). This intron is therefore specific for the protostome lineage.
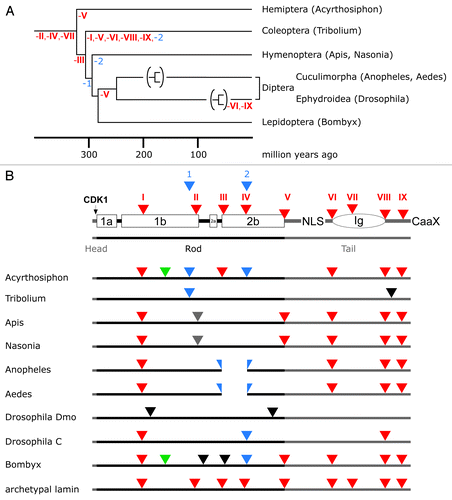
Figure 4. Lamin gene evolution in insects. (A) Phylogenetic tree of insects. The tree and the time scale were taken from reference Citation81. Loss of phylogenetically relevant introns (archetypal in red, arthropod-specific in blue) is indicated below the lines of the tree. For the dipterans only the branches of the Cuculimorpha and the Ephydroidea are shown. (B) Intron patterns of insect lamins. Positions of archetypal introns (red triangles) are shown with respect to lamin protein structure in the top row. Large boxes represent coiled-coil segments. The oval represents the lamin Ig-domain. Head and tail domains are represented by gray bars. Color coding of triangles, green, intron restricted to the protostome lineage; blue, introns restricted to arthropods; gray, introns restricted to hymenopterans (Apis and Nasonia); black, introns unique to individual species. The split blue triangles indicate a deletion of seven heptads in coil 2b of the Anopheles and Aedes lamins.
The Lophotrochozoan Lamin Genes
Not all of the archetypal introns have been retained in all members of the lophotrochozoans, but referred to the group as a whole, all are present. An alignment of lamin protein sequences of two plathelminths, the turbellarian Schmidtea mediterranea and the trematode Schistosoma mansoni, the annelid Capitella teleata and the mollusk Aplysia californica and human lamin B1 is shown in Figure S1. Gain in introns unique for particular species is low.
A hallmark of lamins is the length conservation of the four α-helical sections in the central domain. These α-helical sections show the heptad repeat pattern characteristic for coiled-coil forming proteins. Three of the analyzed lophotrochozoan lamins show a remarkable deviation from this conserved feature. Coil 1b of the Schmidtea, Schistosoma, and Aplysia lamins is shorter by 11 and 9 amino acid residues, respectively (Fig. S1A and B). Lack of these residues has been confirmed in all three cases by cDNA sequence data. Alignment to other lamins reveals that the gap is located approximately mid way in coil 1b. The lack of these residues disrupts the heptad repeat pattern and might result in an additional short linker region in coil 1b.
The Ecdysozoan Lamin Genes
Similar to the lophotrochozoans, all archetypal introns are present in the ecdysozoans, when this group is considered as a whole. The same holds for one of its subgroups, namely the arthropods. However, the ecdysozoans house those species, whose lamin gene structures differ most from that of the archetypal lamin gene. Drosophila and Caenorhabditis are among them. This will become clear when insects and nematodes are considered in detail (see below).
Lamin Genes of Arthropods
The phylum Arthropoda includes several subphyla such as the Chelicerata (including the Xiphosura (horseshoe crabs) as well as the Arachnida (scorpions, spiders and mites), the Crustacea, and the Insecta. The latter is the most species-rich class of the entire animal kingdom. In most arthropods the number of the archetypal introns is larger than in the nematodes (Fig S2, and ). The protostome specific intron in coil 1b (, and ; see also Figs. S1–S3) is retained in the mite Ixodes, the crustacean Daphnia, and in two of the insect species, i.e., Acyrthosiphon and Bombyx (; see also Fig. S3). Coil 1b of the mite lamin is shortened by one heptad. This deletion coincides with an intron border, indicating that this deletion might have occurred by formation of a new splice acceptor site. In coil 2b of the Daphnia lamin eight extra amino acid residues are inserted which might disturb coiled-coil formation. This insertion is not a general feature of crustaceans as in the lamin sequence of another crustacean, the copepod Lepeophtheirus salmonis, it is not found.
Lamin Genes of Insects
In the course of insect diversification more profound changes have taken place. A comparison of eight species from different orders, which include the pea aphid (Acyrthosiphon pisum), two mosquitoes (Aedes aegypti and Anopheles gambiae), the honey bee (Apis mellifera), a parasitoid wasp (Nasonia vitripennis), the red flour beetle (Tribolium castaneum), a fruit fly (Drosophila melanogaster), and a butterfly (Bombyx mori) reveals that the last common ancestor of the insects had lost the archetypal introns II, IV, and VII (). In the arthropod lineage two new introns were gained (blue in ; Figs. S2 and S3). The latter were lost again in the course of insect evolution in some of the lineages. Further loss of archetypal lamin introns differs greatly in the individual lineages. The majority of species retained at least two and up to five of the archetypal introns (; see also Fig. S3). Some species gained one or more new introns that are unique for these species. Strong genetic drift is seen in the lamin gene of Tribolium and the gene encoding lamin Dmo of Drosophila. These two genes have lost all archetypal lamin introns and in Tribolium only one intron matches a position found in another insect species.
The gene structure of the lamin Dmo differs entirely from that of all other species (; see also Fig. S3). It shows the strongest genetic drift of all known lamin genes. The coding region is interrupted by two introns, none of which matches an intron position outside the drosophilids. Within the drosophilids the first intron is conserved in all of the 11 species that were analyzed (data available on request). The second intron is present in all drosophilids except the two members of the obscura group,Citation82 i.e., D. pseudobscura and D. persimilis.
Remarkably, Drosophila has a second lamin called LamC.Citation83 LamC lacks a CaaX motif. Its expression is developmentally regulated.Citation84 Since it shares these two properties with mammalian lamin C, it has been classified as an A-type lamin, while lamin Dmo was classified as a B-type lamin due to its ubiquitous expression.Citation84 From an evolutionary point of view this classification is not justified. Mammalian lamin C is an alternative splicing product of the lamin A gene. The lamin A gene, however, arose only in the course of the vertebrate evolution (see below) and the protein structure of both Drosophila lamins, Dmo and LamC, is that of a B-type lamin. Moreover, the LamC gene shows a greater resemblance to the archetypal lamin gene than the Dmo gene. It houses three introns, two of which correspond to the archetypal introns I and VIII, respectively (; see also Fig. S3). The analogy to the A-type lamins of vertebrates is valid with respect to the lack of CaaX box (mammalian lamin C) and the absence of a (permanent) isoprenylation, in the case of lamin A. In terms of differential expression, it applies only limited because vertebrate lamin B1 is also differentially expressed.Citation85 While lamin C is conserved within the drosophilids (data available on request) no evidence is found that it is present outside the drosophilids. It thus represents a special feature of the fruit flies, which is difficult to put in relation to the occurrence of lamin A in all vertebrates lineages.
Two lamin genes have also been found in two mosquitoes, i.e., Aedes and Anopheles. In contrast to Drosophila, both mosquito lamins, L1 and L2, carry a CaaX motif at their C-termini. Extent of identity between L1 and L2 within the same species is between 53% and 55%; that between the two species is 69% for L1 and 80% for L2. Particularly noteworthy is that all four mosquito proteins have a significantly shorter rod domain. They lack the first seven heptads of coil 2b. The L2 genes contain four of the archetypal lamin introns and an additional new intron. This intron is located at the beginning of coil 2b, at the site where the seven heptads have been deleted (; see also Fig. S3), suggesting that this deletion was caused the by formation of a new splice acceptor site. An EST clone of Aedes (Accession EB088934), which spans the deleted region, proves that this short lamin is indeed expressed. The L1 genes have no introns in their coding regions and might have originated by retrotranscription of processed mRNAs and subsequent insertion into the genome.Citation86
Lamin gene duplication in mosquitoes must have occurred after the deletion of the seven heptads since both genes, L1 and L2, have a short coil 2b. This also shows that the gene duplications have occurred independently in the mosquito and the drosophilid lineages. Gene duplications allow functional specializations (neofunctionalizations). The Drosophila LamC gene acquired an altered membrane targeting due to the lack of lipidation and a restricted, cell type-specific expression.
The Archetypal Pattern of Lamin Introns Has Been Retained to Varying Degrees in the Nematode Lineage
Similarly to the insects, genome drift in the nematode lineage is larger than in basal metazoans or in vertebrates. Within the nematodes intron loss and gain is pronounced (; see also Fig. S4). Of the archetypal lamin introns, three (Loa and Pristionchus), one (Heterodera) or none (Caenorhabditis) have been retained. Two introns (marked in yellow in the Figs.) have been gained in the nematode lineage and all four nematodes have retained the protostome specific intron (marked in green). In addition, all species gained between three and ten (Pristionchus!) new introns, which are unique for the respective species.
Two of the nematode lamins show length variation of their rods. Coil 2 of C. elegans is by two heptads shorterCitation63 and coil 2 of Loa lacks four amino acid residues. The rod domains of Heterodera and Pristionchus are of normal length. The major mitotic phosphorylation site in front of coil 1a, which is one of the hallmarks of lamins, is conserved only in the Loa lamin. The functional impact of this loss in the other nematodes is not known.
The sequence alignment (Fig. S4) includes sequences of four nematodes and the lamin sequence of the priapulid Priapulus caudatus. Priapulids are marine worms, which together with the nematodes are members of the Cycloneuralia. Priapulus has retained two of the nine archetypal introns and did not gain any new ones. The intron in coil 1b common to the lophotrochozoans and nematodes is not present in Priapulus.
Taken together, now that several genomes of species of the respective taxonomic groups have been sequenced, it has become clear that the genome drift in the protostome lineage and particularly in insects and nematodes is larger than in basal metazoans or in vertebrates. Moreover, length variation in the coiled-coil segments of the rod domain is restricted to protostome lamins. But it has also become evident that neither the Drosophila Dmo gene nor the Ce-lam gene, which were the first invertebrate lamin genes to be analyzed, can be taken as representatives for their taxonomic groups.
Lamin Genes of the Deuterostomes
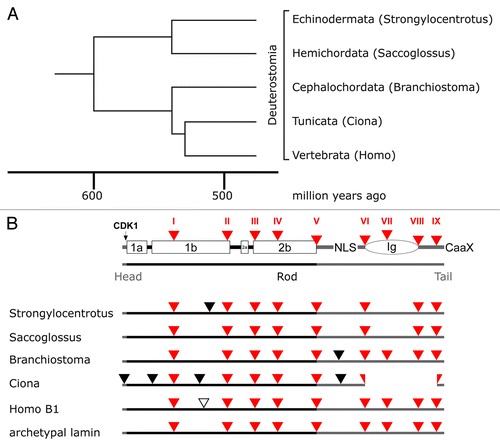
The group of deuterostomes comprises the echinoderms (among them the starfish and the sea urchins), hemichordates, and chordates. The latter includes the subphyla cephalochordates (e.g., lancelets), tunicates (e.g., sea squirts) and the vertebrates (). The lamin gene structure of all these groups largely corresponds to that of the archetypal lamin (; see also Fig. S5). Those introns, which have been gained, are in positions that are not found in any other lamin gene. Strongylocentrotus and Saccoglossus have lost the archetypal intron VII. The tunicate genes underwent a functionally relevant change. They lack the Ig-domain in the tail.Citation87 This loss can be explained by deletion of three exons (; see also Fig. S5) and was confirmed by the analysis of two Ciona species, e.g., Ciona intestinalis and Ciona savignyi. The functional significance of this loss, however, is unclear. The genomes of both species encode a single lamin gene.
Figure 6. Lamin gene evolution in deuterostomes. Phylogenetic tree (A) and intron patterns (B) of deuterostome lamins. The tree and the time scale were taken from reference Citation69. Labeling in (B) is as described in the legend to . The loss of the Ig domain in the tunicates can be explained by deletion of three exons.
Invertebrates Have Only One Lamin Gene
Database searches have identified only a single lamin gene per invertebrate genome so far. We can therefore assume that the additional lamin genes found in the mosquitoes and fruit flies are exceptions rather than the rule for invertebrates.
As representatives of most metazoan phyla have been analyzed, it can be stated that in most phyla the evolution of lamin genes has much more uniformly proceeded as it was initially assumed on the basis of the gene analyses of Caenorhabditis elegans and Drosophila melanogaster. Moreover, the strong genomic drift, which is seen in some nematodes and insects, is also not representative for the entire group of nematodes and insects, respectively.
Lamin Evolution in Vertebrates
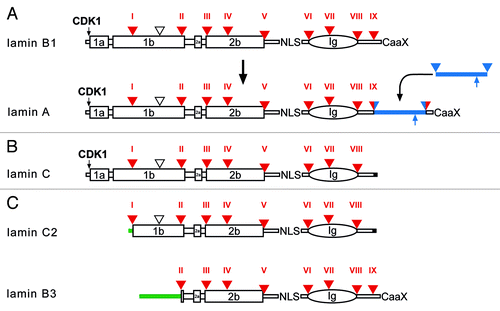
It is hypothesized that two rounds of whole genome duplications have occurred in the ancestral vertebrate.Citation67 The timing of these duplications is constrained to after the divergence of vertebrates from tunicates and lancelets, but before the split between cartilaginous and bony vertebrates.Citation68 For many single genes in invertebrates four vertebrate paralogs are known.Citation88 A prominent example is the Hox cluster of developmental genes.Citation89 This scenario could also explain why vertebrates have four lamin genes, encoding the lamins B1, B2, LIII and lamin A. As mentioned above, vertebrate lamin genes have retained the nine archetypal introns, which are present in basic metazoans. All gained an additional intron, which is positioned in the coil 1b region ( and ). Two further changes have occurred during evolution of the vertebrate lamins: The LMNB2 gene gained an additional intron in the linker 1 regionCitation90 and the tail domain of lamin A was extended by insertion of a new exon into the archetypal intron IXCitation91(). Alignments of lamins B1 and B2 from different vertebrates are given in the Figures S6 and S7, respectively.
Figure 7. Protein structure of vertebrate lamins. (A) A model for the evolution of lamin A from a B-type progenitor. The lamin A specific domain is shown as a blue bar. The ZMPSTE24 cleavage site is indicated by an upward pointing blue arrow. (B) Lamin C, an alternative splice product of the LMNA gene. Lamin C is identical to lamin A up to residue 566 followed by six unique amino acid residues encoded by the 5′-end of intron IX (marked in black). (C) Protein structure of the two mammalian germline-specific lamins, C2 and B3. In lamin C2 the head, coil 1a, and part of coil 1b of lamin C are replaced by a short stretch of amino acid residues (green bar), which serve as myristoylation site. In lamin B3 the head, coil 1a and 1b of lamin B2 are replaced by a new non-α-helical head. The length of the new head differs between rat, mouse, and human between 50 to 81 residues. Intron positions (triangle, and Roman numerals) are shown with respect to protein structure. Large boxes represent coiled-coil segments. The oval represents the lamin Ig-domain. Archetypal intron positions in red, newly gained intron positions in lamin A in red/blue. The vertebrate-specific intron position is marked by an open triangle. NLS, nuclear localization signal; CaaX, isoprenylation motif; CDK1, CDK1 phosphorylation site.
The genomes of fish, amphibians and birds encode all four genes while mammals have lost the LIII gene.Citation92 Transcriptional initiation at different start sites and alternative splicing of primary transcripts gives rise to an even greater number of lamin proteins.Citation93 All vertebrate lamins are differentially expressed, but to a very different extent.Citation94-Citation98
Lamin B2 is nearly universally expressed in somatic cells while lamin B1 expression is more restricted.Citation85,Citation94-Citation97 B1 and B2 are closely associated with the nuclear envelope and remain associated with membranes after disassembly of the nuclear lamina during M-phase.Citation24,Citation99,Citation100 The assumption that lamin B1 and B2 are essential for nuclear integrity, cell proliferation, and developmentCitation36,Citation37,Citation101,Citation102 has recently been challenged by experiments with conditional knockout mice, which show that the absence of lamin B1 and B2 has no effect on cell proliferation of keratinocytes and development of skin and hair.Citation103
The expression pattern of lamin LIII is much more restricted. Observations were made mainly in amphibians and fish.Citation94,Citation95,Citation104 In amphibians LIII is the major lamin of oocytes and early embryos.Citation94,Citation95 In somatic cells it is restricted to a few differentiated cell types.Citation94 EST data indicate that this might also hold true for birds. There are two variants of lamin LIII. They are generated by alternative splicing of two C-terminal CaaX motif-encoding exons. Both LIII proteins are isoprenylated, but differ greatly in their membrane association. LIIIa becomes soluble upon lamina disassembly during M-phase while LIIIb remains membrane associated. For the stable association of LIIIb with membranes six basic amino acid residues and a cysteine residue, which serves as a potential palmitoylation site act as secondary membrane targeting motifs.Citation105 The two alternative exons, and the secondary membrane targeting signals are precisely conserved between fish,Citation104 amphibiansCitation62 and birds,Citation92 which underlines the functional importance of these motifs (Fig. S8). The fact that mammals have lost the lamin LIII gene rather than it has remained undetected has been proven by analysis of the flanking genes.Citation92 While in Xenopus and chicken, the LIII gene is flanked by the genes SH3GL3 and ADAMTSL3, these two genes are directly adjacent on the respective mammalian chromosomes (i.e., chromosome 15 in human, chromosome 7 in mouse, chromosome 1 in rat). Whether LIII is still present in the monotreme platypus (Ornithorhynchus anatinus) would be of particular interest with respect to its function in oocytes. Platypus exhibits a combination of non-mammalian and mammalian characters; for example the females lactate, yet they lay eggs. Like other egg-laying vertebrates, but unlike mammals they produce large vitellogenin-rich eggs. The egg yolk proteins are still present in platypus but have been lost from the eutherian lineage.Citation106 Preliminary results obtained in our group do not preclude that the LIII gene still exists in the platypus genome. However, a definite answer to this question has to await further releases of the platypus genome project.
Lamin A
The structure of lamin A differs from all other lamins. Its tail domain is by about 50 to 100 amino acid residues longer than that of the B-type lamins. These extra amino acid residues are encoded by a single exon, which suggests that the LMNA gene arose by insertion of a new exon in the last intron of a B-type progenitor geneCitation91 (). An alignment of lamin A from different vertebrate species is given in Figure S9. Lamin A is synthesized as prelamin A. After isoprenylation and carboxyl-methylation it undergoes a further proteolytic cleavage by the endoprotease ZMPSTE24, which results in the loss of the hydrophobically modified C-terminus. The lack of a permanent lipidation might explain why mature lamin A becomes soluble during M phase. If for one or another reason there is improper processing of lamin A, this will cause severe diseases, such as premature aging (Hutchinson-Gilford progeria syndrome) or mandibuloacral dysplasia.Citation48,Citation53,Citation57,Citation107 Lamin A is expressed late in development and its appearance is normally correlated with differentiation.Citation85,Citation96,Citation98 It seems to be dispensable for embryonic development but homozygote LMNA KO mice do not survive for more than eight weeks post gestation.Citation108 Lamin A seems to have more specialized functions. It interacts with a large number of nuclear protein partners.Citation109 Filaments formed by lamin A differ from those made of B-type laminsCitation27 and light microscopic analysis indicates that A- and B-type lamins form separate meshworks within the lamina.Citation110 Moreover, experimental evidence reveals that lamin A contributes significantly to nuclear mechanics.Citation31,Citation39,Citation111,Citation112 Its implication in a multitude of cellular functions is highlighted by mutations in the LMNA gene, which cause a broad spectrum of human diseases.Citation25,Citation48,Citation49,Citation52,Citation53 This has made lamin A the most intensely studied of all nuclear envelope proteins.
For the LMNA gene a large number of missense mutations have been described (http://www.umd.be/LMNA/). These are usually found at highly conserved positions. The Lamin A sequences of mammalian species are very similar, e.g., 97–98% of the residues of the dog, pig, and human lamin A are identical (Fig. S9). In dog and pig there is a serine residue at position 602, while there is a glycine in humans. The G602S mutation has been described to cause type A insulin resistance syndrome.Citation113 It is hard to imagine that this serine residue in an otherwise almost identical context represents the wild type in one case and in the other causes a disease. In lamin A proteins, which have a lower degree of identity with the human lamin A, such as the fish lamins, it is less surprising that changes in amino acid residues are found, which cause disease in humans. Lamin A1 of the puffer fish Takifugu contains for example four residues, which in humans cause Emery-Dreifuss muscular dystrophy or dilated cardiomyopathy. Additional examples are found in A-lamins of other fishes.
Lamin A seems to be the evolutionarily most derived member of the vertebrate lamin family. Apart from the lamin A-specific domain in the tail it bears somewhat greater overall resemblance to lamin LIII than to B1 or B2. The following evolutionary scenario might therefore be plausible, regardless of whether the four genes have arisen in the course of two whole genome duplications or by two rounds of selective duplication of the ancestral lamin gene: The first gene duplication led to the formation of two precursor genes. From one of these precursors arose the LMNB1 and LMNB2 gene after the second duplication event. LMNB2 acquired an additional intron (see above). From the other precursor arose the LIII gene and a gene that became the LMNA gene after insertion of the new exon. The insertion of the new exon into an otherwise conserved gene generated a new lamin type. Concomitant with the insertion of the new exon a ZMPSTE24 cleavage site might have been introduced. This would explain the proteolytic processing of prelamin A to the mature protein, a feature unique to lamin A. Processing of prelamin A to lamin A was confirmed experimentally for amphibians, birds and mammals.Citation114-Citation119 At what stage of vertebrate evolution the insertion of the new exon has taken place may be resolved after the completion of genome projects of the elephant shark (Callorhinchus milii), a cartilaginous fish, and the lamprey (Petromyzon marinus). All bony fish analyzed so far have a LMNA gene. Takifugu, Tetraodon (two puffer fishes), and Gasterosterus (stickleback) even have two, which arose from a further gene duplication. The first 20–30 amino acid residues encoded by the lamin A-specific exon are highly conserved in all vertebrate classes while the rest show length variation and little conservation. The shortest lamin A-specific exons are found in fishes.
All mammals express at least one additional A-type lamin, i.e., lamin C. Lamin C is produced as an alternative splice product of the LMNA gene. It is identical to lamin A up to amino acid residue 566 but lacks the residues encoded by exons 11 and 12. Instead, it possesses six unique amino acid residues at its C-terminus (). Importantly, it lacks the CaaX motif and therefore cannot be lipidated. Lamins A and C are the major products of the LMNA gene.Citation15,Citation120,Citation121 They are expressed in nearly all differentiated cells albeit the ratio of the two proteins may differ significantly from cell type to cell type. It should be emphasized that lamin C has not been found outside the mammalian lineage.
In humans a third splice variant of the LMNA gene has been described,Citation120 i.e., lamin AΔ10. Lamin AΔ10 lacks all the residues encoded by exon 10, which basically make up a cluster of acidic residues and a stretch of histidine residues. AΔ10 has been detected in tumor cell lines as well as in several normal tissues.Citation120 Whether it is expressed in other mammals or not, is not yet known.
Germline-Specific Lamins in Amphibians and Mammals
The discovery of germline-specific lamins shows impressively how far diversification of the lamin proteins has progressed in vertebrates. In contrast to somatic vertebrate lamins, which all show an identical structure of the central rod domain, germline-specific lamins have atypical rod domains, which are assumed to affect filament formation and consequently alter the properties of the nuclear lamina. These lamins function in the remodeling of nuclei during meiotic chromosome movements and the morphogenesis of sperm nuclei. The generation of germline-specific lamins follows diverse evolutionary paths in different vertebrate classes.
Lamin LIV of Amphibians
Expression of the amphibian lamin LIV is restricted to male germ cells.Citation122,Citation123 The protein is distributed in a characteristic punctuated pattern along the nuclear envelope of elongated sperm nuclei. The LIV protein is a differential splice product of the lamin LIII gene. The protein contains 40 extra amino acid residues in coil 2b of the rod domain. The extra residues disrupt the heptad repeat pattern of coil 2b and disrupt filament formation.Citation123 Data bank searches gave no hints for the existence of lamin LIV outside the amphibian lineage. It therefore seems that LIV is restricted to amphibians.
Germline-Specific Lamins of Mammals
Mammals express two germline-specific lamins, lamin B3 and lamin C2. Lamin C2 is an alternative splice product of the LMNA gene. In lamin C2, the head and coil 1a of lamin C are replaced by a short non-α-helical stretch of amino acid residues ().Citation124,Citation125 The unique N-terminus serves as a myristoylation site that is essential for nuclear envelope targeting of lamin C2.Citation126
Lamin C2 is expressed in the male germline in spermatocytes and is enriched at telomere attachment sites of meiotic chromosomes.Citation127 It is involved in the remodeling of the nuclear envelope of spermatocytes.
Lamin B3 is an alternative splice product of the LMNB2 gene. In the lamin B3 protein a new non-helical N-terminal domain replaces the head, coil 1a, and 1b of lamin B2 (). The size of the new N-terminal domain differs between different mammalian species. It is specifically expressed in post-meiotic male germ cells. Lamin B3 is ascribed a role in nuclear remodeling during spermiogenesis.Citation128-Citation130 Analysis of the gene structure of the murine Lmnb2 revealed that the B3-specific N-terminus is encoded by a single exon that is located in intron IV of the Lmnb2 gene.Citation123 Data bank searches of a large number of vertebrate genomes make it very unlikely that lamin C2 or B3 are present outside the mammalian lineage.Citation123
Are There Lamins Outside the Metazoans?
Up till now there is no direct evidence of nuclear lamins present outside the metazoans. Neither in the genomes of unicellular eukaryotes (Dictyostelium discoideum), fungi (e.g., Saccharomyces cerevisiae, Laccaria bicolor) nor in those of plants (Arabidopsis thaliana, Physcomitrella patens) lamin sequences have been found. Because the sequence coverage of these genomes is large and the annotations are complete, it can be ruled out that these organisms possess lamins. However, a lamina-like organization at the nuclear envelope has been demonstrated also in non-metazoan eukaryotes. Notably, the first observations were made in unicellular eukaryotes.Citation2-Citation4 Since lamins are involved in key nuclear functions, that are common to all eukaryotes, proteins must exist in the other eukaryotes, which take over these functions. Those nuclear envelope proteins that have been characterized in trypanosomes and plants and that might fulfill the function of lamins show no similarity to lamins, except for the fact that they contain coiled-coil domains.Citation131-Citation134
Recently, a nuclear envelope protein, NE81, has been characterized in the unicellular amoeba Dictyostelium discoideum.Citation135 NE81 has several features in common with lamins. It has a tripartite protein structure with a central α-helical domain, predicted to form coiled-coils. The rod domain is of similar length as that of the lamins. It carries an NLS, a C-terminal CaaX motif, and a CDK1 phosphorylation site in front of the α-helical domain. NE81 is associated with the inner nuclear membrane. The central rod domain of NE81 is subdivided by non-helical linkers. A match of the helix-linker pattern between NE81 and lamins and IFs in general is at least not completely clear. However, as discussed above, some heterogeneity in the rod domains and, more importantly, interruptions of the heptad repeat pattern by deletions of a length that are not equivalent to one or more entire heptads are also found in some metazoans (i.e., protostomes). More important for the classification as a lamin might be the presence or absence of the IF consensus sequences. While the sequence at the N-terminus of the NE81 rod bares some similarity with the corresponding IF-consensus, at the C-terminus no IF-consensus is present. The properties of NE81 justify, however, its denomination as a lamin-like protein; it is the first discovered in an unicellular eukaryote. The future will tell whether it will open the door for discovery of similar proteins in other unicellular eukaryotes or even in plants.
| Abbreviations: | ||
| CaaX | = | C-terminal isoprenylation motif |
| CDK1 | = | Cyclin-dependent kinase 1 |
| IF | = | intermediate filament |
| LMNA | = | human lamin A gene |
| Lmnb2 | = | murine lamin B2 gene |
| NLS | = | nuclear localization signal |
| Rce1 | = | Ras converting enzyme 1 |
| ZMPSTE24 | = | zinc metalloprotease STE24 homolog |
Additional material
Download Zip (400 KB)Acknowledgments
This review is dedicated to Klaus Weber, a pioneer in the field of IF proteins and the one who has contributed many of the results described here. RS is particularly grateful for his support and stimulating discussions over many years. We thank Ketaki Apte and Irm Huttenlauch for critically reading the manuscript. We apologize to those authors whose work has not been cited directly, due to space limitations.
References
- Fawcett DW. On the occurrence of a fibrous lamina on the inner aspect of the nuclear envelope in certain cells of vertebrates. Am J Anat 1966; 119:129 - 45; http://dx.doi.org/10.1002/aja.1001190108; PMID: 6007824
- Pappas GD. The fine structure of the nuclear envelope of Amoeba proteus. J Biophys Biochem Cytol 1956; 2:431 - 4; http://dx.doi.org/10.1083/jcb.2.4.431; PMID: 13357581
- Mercer EH. An electron microscopic study of Amoeba proteus. Proc R Soc Lond B Biol Sci 1959; 150:216 - 32; http://dx.doi.org/10.1098/rspb.1959.0016; PMID: 13633977
- Beams HW, Tahmisian TN, Devine R, Anderson E. Ultrastructure of the nuclear membrane of a gregarine parasitic in grasshoppers. Exp Cell Res 1957; 13:200 - 4; http://dx.doi.org/10.1016/0014-4827(57)90071-X; PMID: 13473864
- Gray EG, Guillery RW. An Electron Microscopical Study of the Ventral Nerve Cord of the Leech. Z Zellforsch Mikrosk Anat 1963; 60:826 - 49; http://dx.doi.org/10.1007/BF00339095; PMID: 14093677
- Coggeshall RE, Fawcett DW. The Fine Structure of the Central Nervous System of the Leech, Hirudo Medicinalis. J Neurophysiol 1964; 27:229 - 89; PMID: 14129772
- Stelly N, Stevens BJ, André J. Étude cytochimique de la lamelle dense de l'enveloppe nucléaire. J Microsc (Paris) 1970; 9:1015 - 28
- Patrizi G, Poger M. The ultrastructure of the nuclear periphery. The zonula nucleum limitans. J Ultrastruct Res 1967; 17:127 - 36; http://dx.doi.org/10.1016/S0022-5320(67)80025-X; PMID: 6017352
- Kalifat SR, Bouteille M, Delarue J. Étude ultrastructurale de la lamelle dense observée au contact de la membrane nucléaire interne. J Microsc (Paris) 1967; 6:1019 - 26
- Aaronson RP, Blobel G. Isolation of nuclear pore complexes in association with a lamina. Proc Natl Acad Sci USA 1975; 72:1007 - 11; http://dx.doi.org/10.1073/pnas.72.3.1007; PMID: 1055359
- Dwyer N, Blobel G. A modified procedure for the isolation of a pore complex-lamina fraction from rat liver nuclei. J Cell Biol 1976; 70:581 - 91; http://dx.doi.org/10.1083/jcb.70.3.581; PMID: 986398
- Gerace L, Blum A, Blobel G. Immunocytochemical localization of the major polypeptides of the nuclear pore complex-lamina fraction. Interphase and mitotic distribution. J Cell Biol 1978; 79:546 - 66; http://dx.doi.org/10.1083/jcb.79.2.546; PMID: 102651
- Aebi U, Cohn J, Buhle L, Gerace L. The nuclear lamina is a meshwork of intermediate-type filaments. Nature 1986; 323:560 - 4; http://dx.doi.org/10.1038/323560a0; PMID: 3762708
- McKeon FD, Kirschner MW, Caput D. Homologies in both primary and secondary structure between nuclear envelope and intermediate filament proteins. Nature 1986; 319:463 - 8; http://dx.doi.org/10.1038/319463a0; PMID: 3453101
- Fisher DZ, Chaudhary N, Blobel G. cDNA sequencing of nuclear lamins A and C reveals primary and secondary structural homology to intermediate filament proteins. Proc Natl Acad Sci USA 1986; 83:6450 - 4; http://dx.doi.org/10.1073/pnas.83.17.6450; PMID: 3462705
- Herrmann H, Aebi U. Intermediate filaments: molecular structure, assembly mechanism, and integration into functionally distinct intracellular scaffolds. Annu Rev Biochem 2004; 73:749 - 89; http://dx.doi.org/10.1146/annurev.biochem.73.011303.073823; PMID: 15189158
- Parry DA, Steinert PM. Intermediate filaments: molecular architecture, assembly, dynamics and polymorphism. Q Rev Biophys 1999; 32:99 - 187; http://dx.doi.org/10.1017/S0033583500003516; PMID: 10845237
- Kapinos LE, Schumacher J, Mücke N, Machaidze G, Burkhard P, Aebi U, et al. Characterization of the head-to-tail overlap complexes formed by human lamin A, B1 and B2 “half-minilamin” dimers. J Mol Biol 2010; 396:719 - 31; http://dx.doi.org/10.1016/j.jmb.2009.12.001; PMID: 20004208
- Parry DAD, Steinert PM. Intermediate filament structure. New York: Springer-Verlag, 1995.
- Herrmann H, Strelkov SV, Feja B, Rogers KR, Brettel M, Lustig A, et al. The intermediate filament protein consensus motif of helix 2B: its atomic structure and contribution to assembly. J Mol Biol 2000; 298:817 - 32; http://dx.doi.org/10.1006/jmbi.2000.3719; PMID: 10801351
- Fuchs E, Weber K. Intermediate filaments: structure, dynamics, function, and disease. Annu Rev Biochem 1994; 63:345 - 82; http://dx.doi.org/10.1146/annurev.bi.63.070194.002021; PMID: 7979242
- Dhe-Paganon S, Werner ED, Chi YI, Shoelson SE. Structure of the globular tail of nuclear lamin. J Biol Chem 2002; 277:17381 - 4; http://dx.doi.org/10.1074/jbc.C200038200; PMID: 11901143
- Krimm I, Ostlund C, Gilquin B, Couprie J, Hossenlopp P, Mornon JP, et al. The Ig-like structure of the C-terminal domain of lamin A/C, mutated in muscular dystrophies, cardiomyopathy, and partial lipodystrophy. Structure 2002; 10:811 - 23; http://dx.doi.org/10.1016/S0969-2126(02)00777-3; PMID: 12057196
- Gerace L, Blobel G. The nuclear envelope lamina is reversibly depolymerized during mitosis. Cell 1980; 19:277 - 87; http://dx.doi.org/10.1016/0092-8674(80)90409-2; PMID: 7357605
- Prokocimer M, Davidovich M, Nissim-Rafinia M, Wiesel-Motiuk N, Bar DZ, Barkan R, et al. Nuclear lamins: key regulators of nuclear structure and activities. J Cell Mol Med 2009; 13:1059 - 85; http://dx.doi.org/10.1111/j.1582-4934.2008.00676.x; PMID: 19210577
- Ben-Harush K, Wiesel N, Frenkiel-Krispin D, Moeller D, Soreq E, Aebi U, et al. The supramolecular organization of the C. elegans nuclear lamin filament. J Mol Biol 2009; 386:1392 - 402; http://dx.doi.org/10.1016/j.jmb.2008.12.024; PMID: 19109977
- Goldberg MW, Huttenlauch I, Hutchison CJ, Stick R. Filaments made from A- and B-type lamins differ in structure and organization. J Cell Sci 2008; 121:215 - 25; http://dx.doi.org/10.1242/jcs.022020; PMID: 18187453
- Nigg EA, Kitten GT, Vorburger K. Targeting lamin proteins to the nuclear envelope: the role of CaaX box modifications. Biochem Soc Trans 1992; 20:500 - 4; PMID: 1397650
- Lutz RJ, Trujillo MA, Denham KS, Wenger L, Sinensky M. Nucleoplasmic localization of prelamin A: implications for prenylation-dependent lamin A assembly into the nuclear lamina. Proc Natl Acad Sci USA 1992; 89:3000 - 4; http://dx.doi.org/10.1073/pnas.89.7.3000; PMID: 1557405
- Krohne G, Waizenegger I, Höger TH. The conserved carboxy-terminal cysteine of nuclear lamins is essential for lamin association with the nuclear envelope. J Cell Biol 1989; 109:2003 - 11; http://dx.doi.org/10.1083/jcb.109.5.2003; PMID: 2808518
- Kaufmann A, Heinemann F, Radmacher M, Stick R. Amphibian oocyte nuclei expressing lamin A with the progeria mutation E145K exhibit an increased elastic modulus. Nucleus 2011; 2; In press http://dx.doi.org/10.4161/nucl.2.4.16119; PMID: 21941106
- Ward GE, Kirschner MW. Identification of cell cycle-regulated phosphorylation sites on nuclear lamin C. Cell 1990; 61:561 - 77; http://dx.doi.org/10.1016/0092-8674(90)90469-U; PMID: 2188730
- Heald R, McKeon F. Mutations of phosphorylation sites in lamin A that prevent nuclear lamina disassembly in mitosis. Cell 1990; 61:579 - 89; http://dx.doi.org/10.1016/0092-8674(90)90470-Y; PMID: 2344612
- Peter M, Nakagawa J, Doree M, Labbe JC, Nigg EA. In vitro disassembly of the nuclear lamina and M phase-specific phosphorylation of lamins by cdc2 kinase. Cell 1990; 61:591 - 602; http://dx.doi.org/10.1016/0092-8674(90)90471-P; PMID: 2188731
- Peter M, Heitlinger E, Häner M, Aebi U, Nigg EA. Disassembly of in vitro formed lamin head-to-tail polymers by CDC2 kinase. EMBO J 1991; 10:1535 - 44; PMID: 1851086
- Harborth J, Elbashir SM, Bechert K, Tuschl T, Weber K. Identification of essential genes in cultured mammalian cells using small interfering RNAs. J Cell Sci 2001; 114:4557 - 65; PMID: 11792820
- Lenz-Böhme B, Wismar J, Fuchs S, Reifegerste R, Buchner E, Betz H, et al. Insertional mutation of the Drosophila nuclear lamin Dm0 gene results in defective nuclear envelopes, clustering of nuclear pore complexes, and accumulation of annulate lamellae. J Cell Biol 1997; 137:1001 - 16; http://dx.doi.org/10.1083/jcb.137.5.1001; PMID: 9166402
- Dahl KN, Kahn SM, Wilson KL, Discher DE. The nuclear envelope lamina network has elasticity and a compressibility limit suggestive of a molecular shock absorber. J Cell Sci 2004; 117:4779 - 86; http://dx.doi.org/10.1242/jcs.01357; PMID: 15331638
- Lammerding J, Fong LG, Ji JY, Reue K, Stewart CL, Young SG, et al. Lamins A and C but not lamin B1 regulate nuclear mechanics. J Biol Chem 2006; 281:25768 - 80; http://dx.doi.org/10.1074/jbc.M513511200; PMID: 16825190
- Lammerding J, Schulze PC, Takahashi T, Kozlov S, Sullivan T, Kamm RD, et al. Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J Clin Invest 2004; 113:370 - 8; PMID: 14755334
- Guelen L, Pagie L, Brasset E, Meuleman W, Faza MB, Talhout W, et al. Domain organization of human chromosomes revealed by mapping of nuclear lamina interactions. Nature 2008; 453:948 - 51; http://dx.doi.org/10.1038/nature06947; PMID: 18463634
- Goldman RD, Gruenbaum Y, Moir RD, Shumaker DK, Spann TP. Nuclear lamins: building blocks of nuclear architecture. Genes Dev 2002; 16:533 - 47; http://dx.doi.org/10.1101/gad.960502; PMID: 11877373
- Levy DL, Heald R. Nuclear size is regulated by importin alpha and Ntf2 in Xenopus. Cell 2010; 143:288 - 98; http://dx.doi.org/10.1016/j.cell.2010.09.012; PMID: 20946986
- Boban M, Braun J, Foisner R. Lamins: ‘structure goes cycling’. Biochem Soc Trans 2010; 38:301 - 6; http://dx.doi.org/10.1042/BST0380301; PMID: 20074079
- Dechat T, Pfleghaar K, Sengupta K, Shimi T, Shumaker DK, Solimando L, et al. Nuclear lamins: major factors in the structural organization and function of the nucleus and chromatin. Genes Dev 2008; 22:832 - 53; http://dx.doi.org/10.1101/gad.1652708; PMID: 18381888
- Wilson KL, Dawson SC. Evolution: functional evolution of nuclear structure. J Cell Biol 2011; 195:171 - 81; http://dx.doi.org/10.1083/jcb.201103171; PMID: 22006947
- Razafsky D, Hodzic D. Bringing KASH under the SUN: the many faces of nucleo-cytoskeletal connections. J Cell Biol 2009; 186:461 - 72; http://dx.doi.org/10.1083/jcb.200906068; PMID: 19687252
- Worman HJ, Ostlund C, Wang Y. Diseases of the nuclear envelope. Cold Spring Harb Perspect Biol 2010; 2:a000760; http://dx.doi.org/10.1101/cshperspect.a000760; PMID: 20182615
- Zaremba-Czogalla M, Dubinska-Magiera M, Rzepecki R. Laminopathies: the molecular background of the disease and the prospects for its treatment. Cell Mol Biol Lett 2011; 16:114 - 48; http://dx.doi.org/10.2478/s11658-010-0038-9; PMID: 21225470
- Worman HJ, Bonne G. “Laminopathies”: a wide spectrum of human diseases. Exp Cell Res 2007; 313:2121 - 33; http://dx.doi.org/10.1016/j.yexcr.2007.03.028; PMID: 17467691
- Wilson KL, Foisner R. Lamin-binding Proteins. Cold Spring Harb Perspect Biol 2010; 2:a000554; http://dx.doi.org/10.1101/cshperspect.a000554; PMID: 20452940
- Maraldi NM, Capanni C, Cenni V, Fini M, Lattanzi G. Laminopathies and lamin-associated signaling pathways. J Cell Biochem 2011; 112:979 - 92; http://dx.doi.org/10.1002/jcb.22992; PMID: 21400569
- Gonzalez JM, Pla D, Perez-Sala D, Andres V. A-type lamins and Hutchinson-Gilford progeria syndrome: pathogenesis and therapy. Front Biosci (Schol Ed) 2011; 3:1133 - 46; PMID: 21622261
- Goldberg MW, Fiserova J, Huttenlauch I, Stick R. A new model for nuclear lamina organization. Biochem Soc Trans 2008; 36:1339 - 43; http://dx.doi.org/10.1042/BST0361339; PMID: 19021552
- Dechat T, Gesson K, Foisner R. Lamina-independent lamins in the nuclear interior serve important functions. Cold Spring Harb Symp Quant Biol 2010; 75:533 - 43; http://dx.doi.org/10.1101/sqb.2010.75.018; PMID: 21209392
- Dechat T, Adam SA, Taimen P, Shimi T, Goldman RD. Nuclear lamins. Cold Spring Harb Perspect Biol 2010; 2:a000547; http://dx.doi.org/10.1101/cshperspect.a000547; PMID: 20826548
- Davies BS, Coffinier C, Yang SH, Barnes RH 2nd, Jung HJ, Young SG, et al. Investigating the purpose of prelamin A processing. Nucleus 2011; 2:4 - 9; http://dx.doi.org/10.4161/nucl.2.1.13723; PMID: 21647293
- Franke WW. Nuclear lamins and cytoplasmic intermediate filament proteins: a growing multigene family. Cell 1987; 48:3 - 4; http://dx.doi.org/10.1016/0092-8674(87)90345-X; PMID: 3791413
- Weber K, Plessmann U, Ulrich W. Cytoplasmic intermediate filament proteins of invertebrates are closer to nuclear lamins than are vertebrate intermediate filament proteins; sequence characterization of two muscle proteins of a nematode. EMBO J 1989; 8:3221 - 7; PMID: 2583097
- Weber K, Plessmann U, Dodemont H, Kossmagk-Stephan K. Amino acid sequences and homopolymer-forming ability of the intermediate filament proteins from an invertebrate epithelium. EMBO J 1988; 7:2995 - 3001; PMID: 3181126
- Dodemont H, Riemer D, Weber K. Structure of an invertebrate gene encoding cytoplasmic intermediate filament (IF) proteins: implications for the origin and the diversification of IF proteins. EMBO J 1990; 9:4083 - 94; PMID: 2249666
- Döring V, Stick R. Gene structure of nuclear lamin LIII of Xenopus laevis; a model for the evolution of IF proteins from a lamin-like ancestor. EMBO J 1990; 9:4073 - 81; PMID: 2249665
- Riemer D, Dodemont H, Weber K. A nuclear lamin of the nematode Caenorhabditis elegans with unusual structural features; cDNA cloning and gene organization. Eur J Cell Biol 1993; 62:214 - 23; PMID: 7925480
- Osman M, Paz M, Landesman Y, Fainsod A, Gruenbaum Y. Molecular analysis of the Drosophila nuclear lamin gene. Genomics 1990; 8:217 - 24; http://dx.doi.org/10.1016/0888-7543(90)90274-X; PMID: 2123469
- Consortium ICGS. Sequence and comparative analysis of the chicken genome provide unique perspectives on vertebrate evolution. Nature 2004; 432:695 - 716; http://dx.doi.org/10.1038/nature03154; PMID: 15592404
- Peter M, Kitten GT, Lehner CF, Vorburger K, Bailer SM, Maridor G, et al. Cloning and sequencing of cDNA clones encoding chicken lamins A and B1 and comparison of the primary structures of vertebrate A- and B-type lamins. J Mol Biol 1989; 208:393 - 404; http://dx.doi.org/10.1016/0022-2836(89)90504-4; PMID: 2795656
- Ohno S. Evolution by gene duplication. Berlin, New York: Springer-Verlag, 1970.
- Putnam NH, Butts T, Ferrier DE, Furlong RF, Hellsten U, Kawashima T, et al. The amphioxus genome and the evolution of the chordate karyotype. Nature 2008; 453:1064 - 71; http://dx.doi.org/10.1038/nature06967; PMID: 18563158
- Erwin DH. Early origin of the bilaterian developmental toolkit. Philos Trans R Soc Lond B Biol Sci 2009; 364:2253 - 61; http://dx.doi.org/10.1098/rstb.2009.0038; PMID: 19571245
- Peterson KJ, Cotton JA, Gehling JG, Pisani D. The Ediacaran emergence of bilaterians: congruence between the genetic and the geological fossil records. Philos Trans R Soc Lond B Biol Sci 2008; 363:1435 - 43; http://dx.doi.org/10.1098/rstb.2007.2233; PMID: 18192191
- Grell KG. Trichoplax adhaerens, F. E. Schulze und die Entstehung der Metazoen. Naturwiss Rundsch 1971; 24:160 - 1
- Srivastava M, Begovic E, Chapman J, Putnam NH, Hellsten U, Kawashima T, et al. The Trichoplax genome and the nature of placozoans. Nature 2008; 454:955 - 60; http://dx.doi.org/10.1038/nature07191; PMID: 18719581
- Silva FB, Muschner VC, Bonatto SL. Phylogenetic position of Placozoa based on large subunit (LSU) and small subunit (SSU) rRNA genes. Genet Mol Biol 2007; 30:127 - 32; http://dx.doi.org/10.1590/S1415-47572007000100022
- Srivastava M, Simakov O, Chapman J, Fahey B, Gauthier ME, Mitros T, et al. The Amphimedon queenslandica genome and the evolution of animal complexity. Nature 2010; 466:720 - 6; http://dx.doi.org/10.1038/nature09201; PMID: 20686567
- Corpet F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res 1988; 16:10881 - 90; http://dx.doi.org/10.1093/nar/16.22.10881; PMID: 2849754
- Henikoff S, Henikoff JG. Amino acid substitution matrices from protein blocks. Proc Natl Acad Sci USA 1992; 89:10915 - 9; http://dx.doi.org/10.1073/pnas.89.22.10915; PMID: 1438297
- Zimek A, Weber K. Flanking genes of an essential gene give information about the evolution of metazoa. Eur J Cell Biol 2011; 90:356 - 64; http://dx.doi.org/10.1016/j.ejcb.2010.10.005; PMID: 21163549
- Lin F, Worman HJ. Structural organization of the human gene (LMNB1) encoding nuclear lamin B1. Genomics 1995; 27:230 - 6; http://dx.doi.org/10.1006/geno.1995.1036; PMID: 7557986
- Zimek A, Weber K. In contrast to the nematode and fruit fly all 9 intron positions of the sea anemone lamin gene are conserved in human lamin genes. Eur J Cell Biol 2008; 87:305 - 9; http://dx.doi.org/10.1016/j.ejcb.2008.01.003; PMID: 18328593
- Halanych KM, Bacheller JD, Aguinaldo AM, Liva SM, Hillis DM, Lake JA. Evidence from 18S ribosomal DNA that the lophophorates are protostome animals. Science 1995; 267:1641 - 3; http://dx.doi.org/10.1126/science.7886451; PMID: 7886451
- Sabater-Muñoz B, Legeai F, Rispe C, Bonhomme J, Dearden P, Dossat C, et al. Large-scale gene discovery in the pea aphid Acyrthosiphon pisum (Hemiptera). Genome Biol 2006; 7:R21; http://dx.doi.org/10.1186/gb-2006-7-3-r21; PMID: 16542494
- Clark AG, Eisen MB, Smith DR, Bergman CM, Oliver B, Markow TA, et al. Evolution of genes and genomes on the Drosophila phylogeny. Nature 2007; 450:203 - 18; http://dx.doi.org/10.1038/nature06341; PMID: 17994087
- Bossie CA, Sanders MM. A cDNA from Drosophila melanogaster encodes a lamin C-like intermediate filament protein. J Cell Sci 1993; 104:1263 - 72; PMID: 8314904
- Riemer D, Stuurman N, Berrios M, Hunter C, Fisher PA, Weber K. Expression of Drosophila lamin C is developmentally regulated: analogies with vertebrate A-type lamins. J Cell Sci 1995; 108:3189 - 98; PMID: 7593280
- Broers JL, Machiels BM, Kuijpers HJ, Smedts F, van den Kieboom R, Raymond Y, et al. A- and B-type lamins are differentially expressed in normal human tissues. Histochem Cell Biol 1997; 107:505 - 17; http://dx.doi.org/10.1007/s004180050138; PMID: 9243284
- Irimia M, Roy SW. Spliceosomal introns as tools for genomic and evolutionary analysis. Nucleic Acids Res 2008; 36:1703 - 12; http://dx.doi.org/10.1093/nar/gkn012; PMID: 18263615
- Riemer D, Wang J, Zimek A, Swalla BJ, Weber K. Tunicates have unusual nuclear lamins with a large deletion in the carboxyterminal tail domain. Gene 2000; 255:317 - 25; http://dx.doi.org/10.1016/S0378-1119(00)00323-1; PMID: 11024292
- Lundin LG, Larhammar D, Hallbook F. Numerous groups of chromosomal regional paralogies strongly indicate two genome doublings at the root of the vertebrates. J Struct Funct Genomics 2003; 3:53 - 63; http://dx.doi.org/10.1023/A:1022600813840; PMID: 12836685
- Venkatesh B, Kirkness EF, Loh YH, Halpern AL, Lee AP, Johnson J, et al. Survey sequencing and comparative analysis of the elephant shark (Callorhinchus milii) genome. PLoS Biol 2007; 5:e101; http://dx.doi.org/10.1371/journal.pbio.0050101; PMID: 17407382
- Zewe M, Höger TH, Fink T, Lichter P, Krohne G, Franke WW. Gene structure and chromosomal localization of the murine lamin B2 gene. Eur J Cell Biol 1991; 56:342 - 50; PMID: 1802718
- Stick R. The gene structure of Xenopus nuclear lamin A: a model for the evolution of A-type from B-type lamins by exon shuffling. Chromosoma 1992; 101:566 - 74; http://dx.doi.org/10.1007/BF00660316; PMID: 1521501
- Zimek A, Weber K. Terrestrial vertebrates have two keratin gene clusters; striking differences in teleost fish. Eur J Cell Biol 2005; 84:623 - 35; http://dx.doi.org/10.1016/j.ejcb.2005.01.007; PMID: 16032930
- Schumacher J, Reichenzeller M, Kempf T, Schnölzer M, Herrmann H. Identification of a novel, highly variable amino-terminal amino acid sequence element in the nuclear intermediate filament protein lamin B(2) from higher vertebrates. FEBS Lett 2006; 580:6211 - 6; http://dx.doi.org/10.1016/j.febslet.2006.10.023; PMID: 17070523
- Benavente R, Krohne G, Franke WW. Cell type-specific expression of nuclear lamina proteins during development of Xenopus laevis. Cell 1985; 41:177 - 90; http://dx.doi.org/10.1016/0092-8674(85)90072-8; PMID: 3888407
- Stick R, Hausen P. Changes in the nuclear lamina composition during early development of Xenopus laevis. Cell 1985; 41:191 - 200; http://dx.doi.org/10.1016/0092-8674(85)90073-X; PMID: 3995581
- Lehner CF, Stick R, Eppenberger HM, Nigg EA. Differential expression of nuclear lamin proteins during chicken development. J Cell Biol 1987; 105:577 - 87; http://dx.doi.org/10.1083/jcb.105.1.577; PMID: 3301871
- Stewart C, Burke B. Teratocarcinoma stem cells and early mouse embryos contain only a single major lamin polypeptide closely resembling lamin B. Cell 1987; 51:383 - 92; http://dx.doi.org/10.1016/0092-8674(87)90634-9; PMID: 3311384
- Röber RA, Weber K, Osborn M. Differential timing of nuclear lamin A/C expression in the various organs of the mouse embryo and the young animal: a developmental study. Development 1989; 105:365 - 78; PMID: 2680424
- Stick R, Angres B, Lehner CF, Nigg EA. The fates of chicken nuclear lamin proteins during mitosis: evidence for a reversible redistribution of lamin B2 between inner nuclear membrane and elements of the endoplasmic reticulum. J Cell Biol 1988; 107:397 - 406; http://dx.doi.org/10.1083/jcb.107.2.397; PMID: 3417755
- Firmbach-Kraft I, Stick R. The role of CaaX-dependent modifications in membrane association of Xenopus nuclear lamin B3 during meiosis and the fate of B3 in transfected mitotic cells. J Cell Biol 1993; 123:1661 - 70; http://dx.doi.org/10.1083/jcb.123.6.1661; PMID: 8276888
- Liu J, Rolef Ben-Shahar T, Riemer D, Treinin M, Spann P, Weber K, et al. Essential roles for Caenorhabditis elegans lamin gene in nuclear organization, cell cycle progression, and spatial organization of nuclear pore complexes. Mol Biol Cell 2000; 11:3937 - 47; PMID: 11071918
- Vergnes L, Peterfy M, Bergo MO, Young SG, Reue K. Lamin B1 is required for mouse development and nuclear integrity. Proc Natl Acad Sci USA 2004; 101:10428 - 33; http://dx.doi.org/10.1073/pnas.0401424101; PMID: 15232008
- Yang SH, Chang SY, Yin L, Tu Y, Hu Y, Yoshinaga Y, et al. An absence of both lamin B1 and lamin B2 in keratinocytes has no effect on cell proliferation or the development of skin and hair. Hum Mol Genet 2011; 20:3537 - 44; http://dx.doi.org/10.1093/hmg/ddr266; PMID: 21659336
- Hofemeister H, Kuhn C, Franke WW, Weber K, Stick R. Conservation of the gene structure and membrane-targeting signals of germ cell-specific lamin LIII in amphibians and fish. Eur J Cell Biol 2002; 81:51 - 60; http://dx.doi.org/10.1078/0171-9335-00229; PMID: 11893082
- Hofemeister H, Weber K, Stick R. Association of prenylated proteins with the plasma membrane and the inner nuclear membrane is mediated by the same membrane-targeting motifs. Mol Biol Cell 2000; 11:3233 - 46; PMID: 10982413
- Warren WC, Hillier LW, Marshall Graves JA, Birney E, Ponting CP, Grutzner F, et al. Genome analysis of the platypus reveals unique signatures of evolution. Nature 2008; 453:175 - 83; http://dx.doi.org/10.1038/nature06936; PMID: 18464734
- Agarwal AK, Fryns JP, Auchus RJ, Garg A. Zinc metalloproteinase, ZMPSTE24, is mutated in mandibuloacral dysplasia. Hum Mol Genet 2003; 12:1995 - 2001; http://dx.doi.org/10.1093/hmg/ddg213; PMID: 12913070
- Sullivan T, Escalante-Alcalde D, Bhatt H, Anver M, Bhat N, Nagashima K, et al. Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy. J Cell Biol 1999; 147:913 - 20; http://dx.doi.org/10.1083/jcb.147.5.913; PMID: 10579712
- Broers JL, Ramaekers FC, Bonne G, Yaou RB, Hutchison CJ. Nuclear lamins: laminopathies and their role in premature ageing. Physiol Rev 2006; 86:967 - 1008; http://dx.doi.org/10.1152/physrev.00047.2005; PMID: 16816143
- Shimi T, Pfleghaar K, Kojima S, Pack CG, Solovei I, Goldman AE, et al. The A- and B-type nuclear lamin networks: microdomains involved in chromatin organization and transcription. Genes Dev 2008; 22:3409 - 21; http://dx.doi.org/10.1101/gad.1735208; PMID: 19141474
- Broers JL, Peeters EA, Kuijpers HJ, Endert J, Bouten CV, Oomens CW, et al. Decreased mechanical stiffness in LMNA−/− cells is caused by defective nucleo-cytoskeletal integrity: implications for the development of laminopathies. Hum Mol Genet 2004; 13:2567 - 80; http://dx.doi.org/10.1093/hmg/ddh295; PMID: 15367494
- Schäpe J, Prausse S, Radmacher M, Stick R. Influence of lamin A on the mechanical properties of amphibian oocyte nuclei measured by atomic force microscopy. Biophys J 2009; 96:4319 - 25; http://dx.doi.org/10.1016/j.bpj.2009.02.048; PMID: 19450502
- Young J, Morbois-Trabut L, Couzinet B, Lascols O, Dion E, Bereziat V, et al. Type A insulin resistance syndrome revealing a novel lamin A mutation. Diabetes 2005; 54:1873 - 8; http://dx.doi.org/10.2337/diabetes.54.6.1873; PMID: 15919811
- Peter A, Stick R. Ectopic expression of prelamin A in early Xenopus embryos induces apoptosis. Eur J Cell Biol 2008; 87:879 - 91; http://dx.doi.org/10.1016/j.ejcb.2008.06.001; PMID: 18675490
- Dagenais A, Bibor-Hardy V, Laliberte JF, Royal A, Simard R. Detection in BHK cells of a precursor form for lamin A. Exp Cell Res 1985; 161:269 - 76; http://dx.doi.org/10.1016/0014-4827(85)90084-9; PMID: 4065220
- Beck LA, Hosick TJ, Sinensky M. Isoprenylation is required for the processing of the lamin A precursor. J Cell Biol 1990; 110:1489 - 99; http://dx.doi.org/10.1083/jcb.110.5.1489; PMID: 2335559
- Weber K, Plessmann U, Traub P. Maturation of nuclear lamin A involves a specific carboxy-terminal trimming, which removes the polyisoprenylation site from the precursor; implications for the structure of the nuclear lamina. FEBS Lett 1989; 257:411 - 4; http://dx.doi.org/10.1016/0014-5793(89)81584-4; PMID: 2583287
- Vorburger K, Kitten GT, Nigg EA. Modification of nuclear lamin proteins by a mevalonic acid derivative occurs in reticulocyte lysates and requires the cysteine residue of the C-terminal CXXM motif. EMBO J 1989; 8:4007 - 13; PMID: 2686979
- Lehner CF, Furstenberger G, Eppenberger HM, Nigg EA. Biogenesis of the nuclear lamina: in vivo synthesis and processing of nuclear protein precursors. Proc Natl Acad Sci USA 1986; 83:2096 - 9; http://dx.doi.org/10.1073/pnas.83.7.2096; PMID: 3515346
- Machiels BM, Zorenc AH, Endert JM, Kuijpers HJ, van Eys GJ, Ramaekers FC, et al. An alternative splicing product of the lamin A/C gene lacks exon 10. J Biol Chem 1996; 271:9249 - 53; http://dx.doi.org/10.1074/jbc.271.16.9249; PMID: 8621584
- Lin F, Worman HJ. Structural organization of the human gene encoding nuclear lamin A and nuclear lamin C. J Biol Chem 1993; 268:16321 - 6; PMID: 8344919
- Benavente R, Krohne G. Change of karyoskeleton during spermatogenesis of Xenopus: expression of lamin LIV, a nuclear lamina protein specific for the male germ line. Proc Natl Acad Sci USA 1985; 82:6176 - 80; http://dx.doi.org/10.1073/pnas.82.18.6176; PMID: 3862126
- von Moeller F, Barendziak T, Apte K, Goldberg MW, Stick R. Molecular characterization of Xenopus lamin LIV reveals differences in the lamin composition of sperms in amphibians and mammals. Nucleus 2010; 1:85 - 95; PMID: 21327107
- Alsheimer M, Benavente R. Change of karyoskeleton during mammalian spermatogenesis: expression pattern of nuclear lamin C2 and its regulation. Exp Cell Res 1996; 228:181 - 8; http://dx.doi.org/10.1006/excr.1996.0315; PMID: 8912709
- Furukawa K, Inagaki H, Hotta Y. Identification and cloning of an mRNA coding for a germ cell-specific A-type lamin in mice. Exp Cell Res 1994; 212:426 - 30; http://dx.doi.org/10.1006/excr.1994.1164; PMID: 8187835
- Alsheimer M, von Glasenapp E, Schnolzer M, Heid H, Benavente R. Meiotic lamin C2: the unique amino-terminal hexapeptide GNAEGR is essential for nuclear envelope association. Proc Natl Acad Sci USA 2000; 97:13120 - 5; http://dx.doi.org/10.1073/pnas.240466597; PMID: 11078531
- Alsheimer M, von Glasenapp E, Hock R, Benavente R. Architecture of the nuclear periphery of rat pachytene spermatocytes: distribution of nuclear envelope proteins in relation to synaptonemal complex attachment sites. Mol Biol Cell 1999; 10:1235 - 45; PMID: 10198069
- Furukawa K, Hotta Y. cDNA cloning of a germ cell specific lamin B3 from mouse spermatocytes and analysis of its function by ectopic expression in somatic cells. EMBO J 1993; 12:97 - 106; PMID: 8094052
- Schütz W, Benavente R, Alsheimer M. Dynamic properties of germ line-specific lamin B3: the role of the shortened rod domain. Eur J Cell Biol 2005; 84:649 - 62; http://dx.doi.org/10.1016/j.ejcb.2005.03.001; PMID: 16106909
- Schütz W, Alsheimer M, Ollinger R, Benavente R. Nuclear envelope remodeling during mouse spermiogenesis: postmeiotic expression and redistribution of germline lamin B3. Exp Cell Res 2005; 307:285 - 91; http://dx.doi.org/10.1016/j.yexcr.2005.03.023; PMID: 15950617
- Masuda K, Xu ZJ, Takahashi S, Ito A, Ono M, Nomura K, et al. Peripheral framework of carrot cell nucleus contains a novel protein predicted to exhibit a long alphα-helical domain. Exp Cell Res 1997; 232:173 - 81; http://dx.doi.org/10.1006/excr.1997.3531; PMID: 9141634
- Dittmer TA, Stacey NJ, Sugimoto-Shirasu K, Richards EJ. LITTLE NUCLEI genes affecting nuclear morphology in Arabidopsis thaliana. Plant Cell 2007; 19:2793 - 803; http://dx.doi.org/10.1105/tpc.107.053231; PMID: 17873096
- Meier I, Phelan T, Gruissem W, Spiker S, Schneider D. MFP1, a novel plant filament-like protein with affinity for matrix attachment region DNA. Plant Cell 1996; 8:2105 - 15; PMID: 8953774
- Rout MP, Field MC. Isolation and characterization of subnuclear compartments from Trypanosoma brucei. Identification of a major repetitive nuclear lamina component. J Biol Chem 2001; 276:38261 - 71; PMID: 11477078
- Krüger A, Batsios P, Baumann O, Luckert E, Schwarz H, Stick R, et al. Characterization of NE81, the first lamin-like nucleoskeleton protein in a unicellular organism. Mol Biol Cell 2012; 23:360 - 70; http://dx.doi.org/10.1091/mbc.E11-07-0595; PMID: 22090348
- Regier JC, Shultz JW, Zwick A, Hussey A, Ball B, Wetzer R, et al. Arthropod relationships revealed by phylogenomic analysis of nuclear protein-coding sequences. Nature 2010; 463:1079 - 83; http://dx.doi.org/10.1038/nature08742; PMID: 20147900
- Riemer D, Weber K. The organization of the gene for Drosophila lamin C: limited homology with vertebrate lamin genes and lack of homology versus the Drosophila lamin Dmo gene. Eur J Cell Biol 1994; 63:299 - 306; PMID: 8082654
- Erber A, Riemer D, Hofemeister H, Bovenschulte M, Stick R, Panopoulou G, et al. Characterization of the Hydra lamin and its gene: A molecular phylogeny of metazoan lamins. J Mol Evol 1999; 49:260 - 71; http://dx.doi.org/10.1007/PL00006548; PMID: 10441677