Abstract
Nesprin-2, a type II transmembrane protein of the nuclear envelope, is a component of the LINC complex that connects the nuclear lamina with the actin cytoskeleton. To elucidate its physiological role we studied wound healing in Nesprin-2 Giant deficient mice and found that a loss of the protein affected wound healing particularly at later stages during fibroblast differentiation and keratinocyte proliferation leading to delayed wound closure. We identified altered expression and localization of transcription factors as one of the underlying mechanisms. Furthermore, the actin cytoskeleton which surrounds the nucleus was altered and keratinocyte migration was slowed down and focal adhesion formation enhanced. We also uncovered a new activity of Nesprin-2. When we probed for an interaction of Nesprin-2 Giant with chromatin we observed in ChIP Seq experiments an association of the protein with heterochromatic and centromeric DNA. Through this activity Nesprin-2 can affect the nuclear landscape and gene regulation. Our findings suggest functions for Nesprin-2 at the nuclear envelope (NE) in gene regulation and in regulation of the actin cytoskeleton which impact on wound healing.
Introduction
Nesprins are a family of nuclear membrane proteins characterized by spectrin repeats and a C-terminal transmembrane domain, the KASH-domain, which anchors them in the nuclear membrane. The four members of the family connect the nucleus through their N-termini to the actin cytoskeleton (Nesprin-1 and -2), to the intermediate filament system (Nesprin-3) and to the microtubule network (Nesprin-2 and -4).Citation1-Citation5 The Nesprin-1 and -2 genes, SYNE-1 and SYNE-2, give rise to several isoforms.Citation6 The longest ones, Nesprin-1G and -2G, harboring an N-terminal α-actinin like actin-binding domain (ABD) localize to the outer and inner nuclear membrane.Citation7
Nesprins are components of the LINC complex (linker of nucleoskeleton and cytoskeleton). They interact through their KASH-domain with the C-termini of SUN-domain proteins in the perinuclear space forming the core of a protein assembly which connects the nuclear interior with cytoskeletal components in the cytoplasm.Citation8 Like the Nesprins, SUN domain proteins are type II integral transmembrane proteins with a C-terminus facing the perinuclear space and an N-terminus reaching into the nucleoplasm. Both Nesprins and SUN proteins interact with Lamin A/C, intermediate filament proteins that form a network underneath the inner nuclear membrane, and have crucial functions in maintaining nuclear architecture, regulating the organization of chromosomes, transcription, cell cycle progression and nuclear migration.Citation9 Further interaction partners of Nesprins at the NE are Emerin, muscle A-kinase anchoring protein (mAKAP), muscle-specific tyrosine kinase (MuSK) and kinesin light chain. The respective binding sites have been mapped to C-terminal spectrin repeats of the Nesprins.Citation1,Citation7,Citation10-Citation13
Recent studies suggested that Nesprin-2 is also a component of signaling platforms at the NE and in the nucleus. At the NE it interacts with α- and β-catenin and with Emerin regulating WNT-signaling, in the nucleus KASH-less isoforms interact with ERK1/2 kinases and affect signaling.Citation12
Nesprin-1 and -2 are widely expressed and their expression overlaps in several organs like stomach, kidney and spleen as well as in cell lines. In brain, heart and skeletal muscle Nesprin-1 is the more abundant protein, whereas in lymphoid and hematopoietic tissues, in liver and in skin Nesprin-2 is the predominant protein and in skin mainly the largest isoform is expressed whereas the C-terminal isoforms are hardly detectable.Citation3,Citation18
Mutations in nuclear envelope proteins like Lamins, Emerin or Nesprins lead to severe human diseases collectively known as laminopathies or nuclear envelopathies and include progeria, neuropathy and lipodystrophy to cardiac and muscular dystrophies.Citation14 Most of them are due to mutations in the LMNA gene encoding Lamin A/C. Although Lamins A/C are expressed in virtually all somatic cells, mutations in these proteins manifest in phenotypes that differ with respect to the affected tissue, the age of onset and the severity. The underlying mechanisms may be alterations of the mechanical stability of the cells, altered gene transcription or altered signaling processes.Citation15 More recently, components of the LINC complex have been discussed in this context as disruption of the complex can result in alterations in cellular structure and function that may contribute to the development of laminopathies. In fact, mutations in Nesprin-1 and Nesprin-2 were shown to be responsible for some cases of Emery-Dreifuss muscular dystrophy.Citation16,Citation17
Previously we reported the generation of mice lacking the largest Nesprin-2 isoform Nesprin-2G (Nesprin-2 knockout mice). For the inactivation of the SYNE-2 gene a vector was used that targeted exons encoding part of the actin-binding domain. This resulted in the interruption of the reading frame leading to the complete loss of Nesprin-2 Giant. The protein was no longer detectable by protein gel blot analysis and in immunofluorescence analysis. Smaller C-terminal isoforms that arise from the use of internal transcription or alternative translation sites were still present in those tissues where they are expressed.Citation18 The knock out (KO) mice are viable and healthy. Nesprin-2 KO mice have also been reported by Zhang et al.Citation19 in which the C-terminal isoforms were specifically targeted. Similarly the mice were viable and did not exhibit obvious changes.
As Nesprin-2 Giant is strongly expressed in skin we focused on this organ in our further analysis of the knockout animals. Important also, Nesprin-2 Giant is the major Nesprin-2 isoform in skin whereas C-terminal isoforms are detected at very low levels in skin lysates with an antibody generated against a polypeptide encompassing the last four spectrin repeats of Nesprin-2Citation7 and appear as ~40 and 50 kDa proteins.Citation18 We observed a thickening of the epidermis as a consequence of increased epithelial nuclear size and a relocalization of Emerin from the NE to the cytoplasm. Nuclei in primary dermal knockout fibroblasts and keratinocytes were often misshapen displaying a striking similarity to nuclear deformations characteristic for laminopathies. In vitro wound healing was impaired in Nesprin-2 KO fibroblasts, the cells had a slower migration rate and cell polarization was affected. Furthermore, Lamin A/C organization was perturbed.Citation18 Here we performed in vivo wound healing experiments with which we could probe Nesprin-2's role in cell proliferation, differentiation and migration. The results suggest that Nesprin-2 affects tissue regeneration in wound repair by regulating the cytoskeleton and affecting signaling processes.
Results
Loss of Nesprin-2G results in altered wound repair and defects in cell migration, proliferation and differentiation
Based on the prominent expression of Nesprin-2G in the epidermis (Fig. S1A) and dermis we tested the response of the mutant skin to an injury. Macroscopic inspection of KO wounds revealed a slight acceleration in wound closure during the initial time points. In contrast, after 7 and 10 d there was a significantly delayed wound closure. The macroscopic findings were confirmed by histological analysis where from day 7 onwards the distance between the migrating epidermal tips was increased in sections from KO mice compared with wild type (WT) sections, and at days 7 and 10 the distance between the migrating epidermal tips in the KO was significantly larger than in the WT ().
Figure 1. Wound healing is delayed in Nesprin-2 Giant knock out mice and the macrophage population is increased in KO mice. (A) Wounds from WT and Nesprin-2 KO mice at day (D) 0 and 10. The dotted red line indicates the wound area left open at day 10 (10D). (B) Graph showing progress in wound closure in WT and Nesprin-2 KO mice. At each time point wounds from WT and KO mice (n = 2 wounds/mouse; six mice per time point) were analyzed and the percentage (%) of the open wound area was calculated from macroscopic observation (*p < 0.05). (C) Skin sections of WT and KO were stained with HE (shown for day 1 and 10). The position of the migrating epithelial tip is indicated by arrowheads. (D) Distance between the migrating epidermal tips during wound healing. At day 7 and 10 the distance differs significantly in WT and KO (n = 3–6 sections/wound/mouse, *p < 0.05 and **p < 0.01). (E) Detection of macrophages with F4/80 antibody. Brown staining indicates F4/80 stained cells (shown for day 5). (F) Quantitative PCR analysis shows significant upregulation of chemokine MCP1/CCL2 in KO skin compared with WT at all three time points (n = 6, *p < 0.05 and **p < 0.01; fold changes are given in arbitrary units).
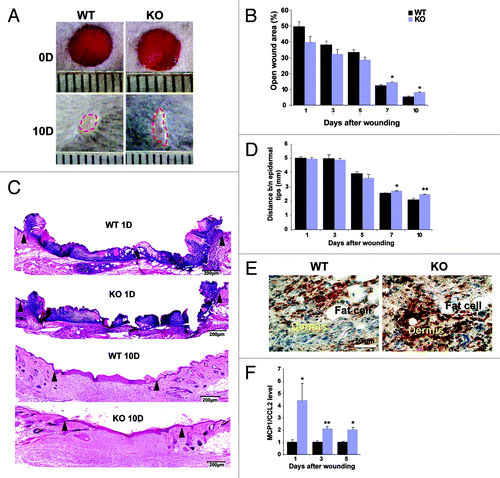
For a detailed analysis of the initial phase of wound healing, the inflammatory phase, we stained the wound tissues for the macrophage marker F4/80 in order to detect infiltration of macrophages. At day 1, 3 and 5 more F4/80 positive cells were seen in sections from KO wounds (, brown staining, shown for day 5) possibly explaining the slightly enhanced wound closure in the KO animals during the early stages. Since macrophages are attracted by chemokines like MCP-1/CCL2 (monocyte chemoattractant protein-1/chemokine ligand 2), we tested MCP-1 expression in the wounds. In an earlier microarray analysis of RNA derived from dermal fibroblasts of Nesprin-2G KO and WT mice we had observed an upregulation of MCP-1 (data not shown). Performing quantitative real time PCR (qRT-PCR) analysis we observed significantly increased expression levels of MCP-1 in KO wounds at days 1, 3 and 5 confirming our F4/80 staining results ().
Migration of keratinocytes toward the wound area occurs during the new tissue formation phase followed by hyper-proliferation of the keratinocytes. We studied the keratinocyte migration pattern by staining the sections from 5, 7 and 10 d after wounding for Keratin 14 (K14) as a marker for keratinocyte differentiation (). In case of WT wounds from day 5 and 7 the keratinocytes (red staining) migrate from the neighboring epidermis toward the wound area and migrate further between the fibrin clot and the dermis by forming a sharp edge (indicated by the dotted line in ) also called migrating tongue. In Nesprin-2 KO wounds keratinocytes do not show such a migration pattern in both 5 and 7 d samples. The absence of a migrating tongue could be one of the reasons for slower wound closure in the KO mice. At day 10, the wounds in the WT were sealed, and the epidermis at the wound area was thin and resembled normal skin. The white line (day 10) indicates the wounded epidermis between the migrating tongues. In KO wounds at day 10 the distance between the migrating tongues differed from that of WT and also the epidermis was much thicker indicating that the wound in KO has not completely closed.
Figure 2. Nesprin-2 KO wounds show altered epithelium formation and reduced proliferation during wound re-epithelialization. (A) Keratinocyte migration over the wound area between fibrin and the dermis was studied with keratinocyte differentiation marker K14 (a cytokeratin 14 specific antibody). Red, K14 staining, blue, DAPI staining of the nucleus. Scale bar, 100 μm. White line in the 10D panels indicates the distance between the migrating tips. Epi, epidermis; Derm, dermis. (B) Sections from WT and Nesprin-2 KO wounds (5, 7 and 10 d after wounding) were stained with a Ki-67 specific antibody as marker for keratinocyte proliferation. The examples shown are from 10D. (C) Quantification of Ki-67 positive cells. Cells positive for Ki-67 were counted per unit area. KO wounds showed significantly reduced numbers of Ki-67 positive cells (red) compared with WT at 5, 7 and 10 d after wounding (n = 2–3 sections per animal, four animals per time point per strain) (*p < 0.05). (D) Decrease in cell population in Nesprin-2 KO. Fibroblasts from WT and KO (passage 2 and 3) were cultured on culture plates (n = 100 cells) and their proliferation rate was measured as increase in cell population after 48 h.
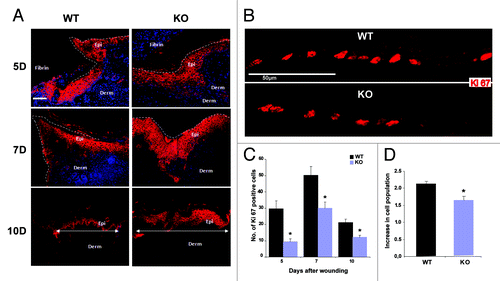
Keratinocyte migration and proliferation are crucial events for reepithelialisation of the wound and alterations in these processes might be causing the observed delay. Keratinocyte proliferation was assessed by staining for the cell proliferation marker Ki-67. Counting the Ki-67 positive cells revealed a reduction in their number in the KO at all time points tested (, bright red stained cells; ). We next determined whether the reduced keratinocyte proliferation leads to reduced hyperproliferative epidermis in the KO and found in our measurements that the length and the area of the hyperproliferative epidermis in hematoxylin and eosin (HE) stained sections was indeed reduced (Fig. S2A and B). Such a reduction leads in general to slower re-epithelialization as we observed in the KO wounds.
The reduction in proliferation was not limited to keratinocytes in the in vivo situation but was also found for primary fibroblasts in culture. After 48 h of culturing, fibroblasts from KO showed only a 1.65 times increase in cell numbers as compared with 2.12 times for WT (). From these observations we propose that, Nesprin-2 is required for the cell proliferation process.
Restoration of the dermal matrix requires the migration and proliferation of fibroblasts and overlaps with reepithelialisation. Fibroblasts in the dermis synthesize extracellular matrix to strengthen the damaged tissue and subsequently to contract the granulation tissue. For wound contraction, the fibroblasts differentiate into specialized cells called myofibroblasts which are characterized by stress fibers containing α smooth muscle actin (α-SMA).Citation20,Citation21 When we investigated the expression of α-SMA in the wounds of KO and WT mice by immunofluorescence analysis and quantified the pixel intensity of staining per unit area we noticed differences at day 5 and a significantly higher intensity of α-SMA staining per unit wound area in WT at days 7 and 10 indicating that more fibroblasts had differentiated into myofibroblasts in WT than in Nesprin-2G KO (). To study wound contraction in more detail we measured the area of the granulation tissue. This is a highly vascularised tissue which replaces the initial fibrin clot in the wound and which can be revealed by Sirius red staining. We observed a larger area at day 7 and 10 in Nesprin-2G KO wounds being indicative of delayed wound healing too ().
Figure 3. Differentiation of fibroblasts into myofibroblasts is reduced during the wound healing process in Nesprin-2 KO mice. (A) Differentiated myofibroblasts were identified by staining for α smooth muscle actin (α-SMA) as shown for day 7. Scale bar, 100 μm. (B) α-SMA staining was quantified as intensity per unit area. The intensity per unit area at 7 and 10 d after wounding differed significantly (*p < 0.05) (n = 2–3 sections per animal, 4–5 animals per time point per strain) between WT and Nesprin-2 KO mice. (C) Granulation tissue area was measured at 7 and 10 d (**p < 0.001). Larger granulation tissue area indicates slower wound contraction (n = 2–3 sections per animal, six animals per time point per strain). (D) Increased distance between the panniculus carnosus (PC) in KO wounds is also indicative of retarded wound contraction (*p < 0.05) (n = 2–3 sections per animal, 6 animals per time point per strain).
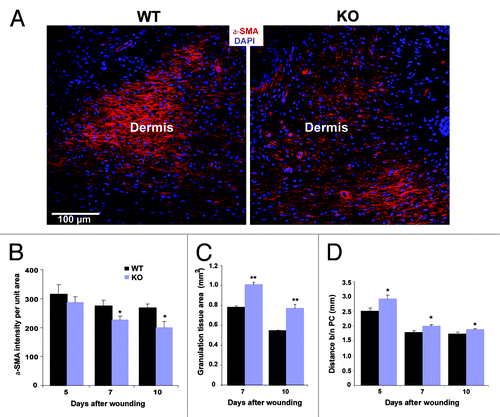
For the wound healing experiment four circular full thickness wounds, that is beyond the panniculus carnosus (PC) muscle, were made on the dorsal side of the mice. The PC contributes to the wound healing process by contracting the wound, and excision of the PC muscle delays wound healing.Citation22 When we determined the distance between the edges of the PC to get a measurement for wound contraction we found that it is decreased in WT and KO wounds over time, however, in KO wounds this distance was significantly wider compared with WT (). All these deficiencies will contribute to slower wound healing and contraction in the Nesprin-2 KO.
Next we wanted to know by which mechanisms Nesprin-2 affects these processes and analyzed cytoskeleton dependent properties of the cells and a potential involvement of Nesprin-2 in signaling processes.
Nesprin-2G deficient fibroblasts have an altered cytoskeleton
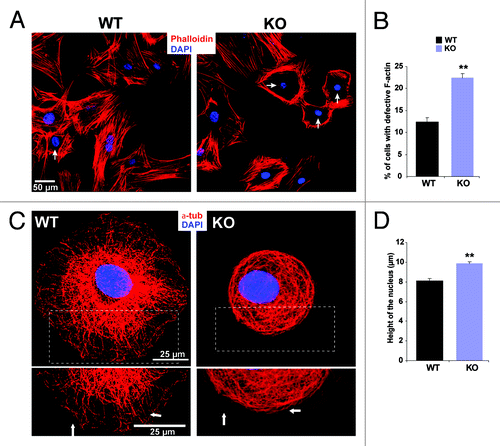
A dynamic actin cytoskeleton is critical for numerous cellular processes including cell adhesion, migration and cytokinesis. These processes require a precise regulation of cell shape and mechanical force generation which is dependent on rearrangements in the F-actin network. Nesprin-2G can bind to F-actin through its N-terminal ABD. The ABD also affected actin polymerization kinetics by shortening the elongation time which led to an increased rate of actin polymerization in a concentration dependent manner.Citation2 F-actin staining in Nesprin-2G KO fibroblasts derived from uninjured dermis revealed that filaments were frequently less regular around the nucleus and mostly absent from the area over the nucleus (Vids. S1 and S2). In WT cells we mainly observed the typical distribution of F-actin throughout the cell (). Approximately 22% of Nesprin-2G KO fibroblasts exhibited a defective F-actin pattern, whereas in WT it was ~12% (). The microtubule system was altered as well. In WT cells microtubules extended outward toward the cell periphery whereas in the KO cells microtubules were arranged in a circular pattern (). An effect on the microtubule system is not unexpected as we recently have reported an interaction of Nesprin-2 with the microtubule motor protein kinesin.Citation13 Shape and positioning of the nucleus largely depend on the cytoskeleton and alterations of the LINC complex lead to changes.Citation23,Citation24 We quantified the nuclear height by carrying out Z-stack imaging of stained nuclei with a laser scanning confocal microscope. In WT we measured 8.1 ± 1.64 μm for the nuclear height, in KO primary fibroblasts the height increased to 9.85 ± 1.64 μm ().
Figure 4. Nesprin-2 maintains actin fibers around the nucleus in fibroblasts. (A) WT fibroblasts and Nesprin-2 KO fibroblasts were stained for F-actin with TRITC Phalloidin. DAPI is used for staining the nucleus. The arrow points to an altered cytoskeleton. (B) Fibroblasts with altered actin fiber arrangement (arrows) were counted and calculated as percent of total fibroblasts. Seven-hundred seventy-seven WT and 991 Nesprin-2 KO cells were used for the analysis. KO fibroblasts showed a significantly higher number of cells with a defective actin cytoskeleton (*p < 0.0001). (C) The microtubule system is altered in Nesprin-2 KO fibroblasts. Cells were stained with mAb WA3 for α-tubulin. Arrows point to the organization of microtubules in the cell periphery. (D) The nuclear height is increased in Nesprin-2G deficient cells. DAPI was used to stain the nuclei. Ninety-six WT and 102 KO cells were analyzed (**p < 0.001).
Cell migration defects in mutant primary keratinocytes are associated with altered focal adhesions
To ask whether altered keratinocyte migration contributes to the wound healing defects, we analyzed the migratory behavior of keratinocytes after introduction of scratch wounds into monolayers (). Whereas WT keratinocytes had significantly migrated toward the scratch after 48 h, the open wound area in the KO was wider. Quantification of the speed of migration supported this difference revealing a slower migration for KO keratinocytes [WT, 4.27 ± 1.33, KO, 1,66 ± 0.48 μm/h (12 h); WT, 3,20 ± 0.53, KO, 0.91 ± 0.29 (24 h) and WT, 2.92 ± 0.38, KO, 0.69 ± 0.10 (48 h)] (). Pictures taken at different time points using time lapse video microscopy confirmed these observations.
Figure 5. Migration and focal adhesion formation is altered in Nesprin-2G deficient keratinocytes. (A) A scratch-wound healing assay reveals a migration defect in Nesprin-2G deficient primary keratinocytes. Wound closure was followed by live cell microscopy (0 to 48 h after scratching). Scale bar, 200 µm. (B) The speed of migration was determined at the indicated time points (µm/hr). (C) Formation of focal adhesions in control and Nesprin-2G deficient primary keratinocytes. Keratinocytes were trypsinized and plated onto type I collagen. Focal adhesion formation was assessed after 3.5 and 6.5 h by staining for Vinculin. The boxed area shows focal contacts (arrows). Scale bar, 25 μm. (D) Determination of the percentage of cells that had formed focal adhesions 3.5 and 6.5 h after plating. 285 WT cells were analyzed each at both time points and 100 (3.5 h) and 130 (6.5 h) KO cells. (E) Determination of the area containing cells positive for Vinculin. 111 WT and 60 KO keratinocytes were examined. (F) protein gel blot analysis showing the expression of Vinculin protein in Nesprin-2 KD and WT cells. Actin was used as a loading control.
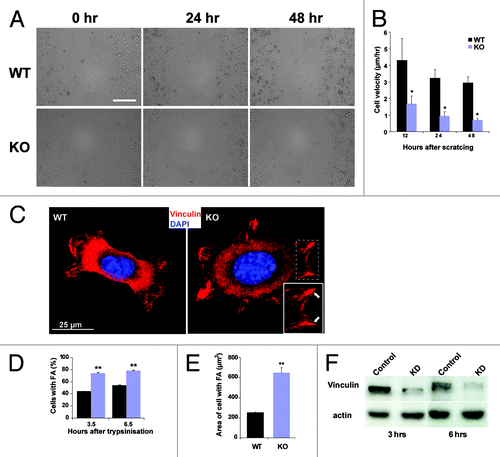
As an altered cell migration is often associated with altered cell adhesion propertiesCitation23 we explored the formation and size of focal adhesions. For that cells were immunostained with Vinculin specific antibodies at 3.5 and 6.5 h after trypsinization and replating onto a type I collagen matrix. Significantly more KO keratinocytes had formed focal adhesions at both time points and the cells appeared more spread. In accordance, the area of the cells on the substratum was increased in comparison to WT (). Support for enhanced focal adhesion formation comes also from experiments with HaCaT keratinocytes in which Nesprin-2 amounts were reduced by siRNA. For this we transfected the cells with a plasmid encoding a shRNA targeted to the C terminus of Nesprin-2 or a control. Loss of Nesprin-2 was confirmed both by immunofluorescence and protein gel blot analysis using pAbK1 which recognizes a C-terminal epitope (Fig. S1B).Citation7 The knock down (KD) cells formed large focal contacts as determined by measuring their length and area (Fig. S3A and B). For Nesprin-2 KD the length measurements were 2.47 µm ± 1.45, for WT cells 2.13µm ± 1.22. In parallel the area of focal contacts was also increased in KD cells (3.02 µm2 ± 2.26) as compared with WT (2.46 µm2 ± 1.95). As we found that area and length of focal adhesion and focal contacts are enhanced in KO and KD cells in comparison to WT cells we further analyzed whether KO and KD cells do not only have the capacity to spread faster but also express increased amounts of focal adhesion markers. Surprisingly, protein gel blot analysis revealed that Nesprin-2 KD cells express less Vinculin at 3 and 6 h (; both at 3 and 6 h after replating). Actin was used as a loading control.
Transcription factors show alterations in KO wounds
The NE is not only a border between the nucleoplasm and the cytoplasm; it also has roles in gene expression through interactions of its components with chromatin. In general, genes organized in chromatin which is associated with the nuclear lamina are typically repressed.Citation25 So far NE-chromatin interactions were mainly shown for Lamin A/C and Lamin associated proteins; however, other components of the NE like BAF and the SUN proteins can also bind to chromatin.Citation26,Citation27 Another possibility for the NE to influence gene expression is by binding to and sequestration of transcription factors.Citation28
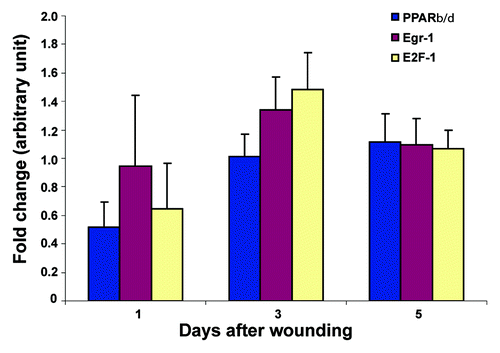
We first tested the effect of the loss of Nesprin-2G on gene expression and selected transcription factors that are known regulators of the wound healing process, namely PPARβ/δ (peroxisome proliferator-activator receptor β/δ), EGR-1 (early growth response factor 1) and E2F1.Citation29-Citation31 PPAR β/δ regulates keratinocyte differentiation during both normal epidermis renewal and re-epithelialisation of skin wounds, EGR-1 can be induced at sites of injury and repair and regulates the transcription of a wide array of downstream genes involved in inflammation, matrix formation, and remodeling, and E2F1 has a unique role in keratinocyte proliferation, adhesion, and migration as well as in wound repair and epidermal regeneration in vivo. We found that expression of PPARβ/δ and E2F1 was downregulated in wounds from KO at day 1 compared with the respective time points from WT. Expression of EGR-1 and E2F1 was slightly increased in KO wounds at day 3. At day 5 no difference in expression level was found ().
Figure 6. Expression of transcription factors differs in Nesprin-2 KO wounds. The expression of transcription factors PPAR-β/δ, EGR-1 and E2F1 was studied by qRT-PCR using RNA from wounds harvested at the indicated time points and the KO/WT ratio of mRNA for the respective transcription factors determined (five mice each from WT and KO per time point). Mean values and standard errors are shown.
Nesprin-2 participates in TGFβ-induced SMAD signaling
TGFβ is an important regulator of wound healing affecting different processes during repair.Citation32 It activates Smad3, which then inhibits AP-1-mediated induction of PPARβ/δ. This results in downregulation of PPARβ/δ at the wound site. Activation of Smad proteins occurs through phosphorylation followed by translocation to the nucleus.Citation33,Citation34 We investigated whether Nesprin-2 is involved in these signaling processes in HaCaT KD cells (; Fig. S1B). Signaling was induced by addition of TGFβ to shRNA treated KD and control cells and expression and localization of SMAD2/3 was followed by immunofluorescence analysis. In control cells, SMAD 2/3 staining intensity increased from the 5 min time point onwards in parallel with an enrichment in the nucleus reaching high levels after 30 min of treatment (). In contrast, in Nesprin-2 KD cells translocation of SMAD2/3 was slower and first seen after 10 min. For each time point we analyzed ~500 cells and determined the nuclear vs. cytosolic distribution of SMAD2/3. With this analysis we could confirm delayed nuclear accumulation of SMAD2/3 in the Nesprin-2 KD cells. The difference was statistically significant (; p < 0.05).
Figure 7. Nesprin-2 mediates the translocation of SMAD 2/3 after TGFβ induction. (A) Translocation of SMAD2/3 was studied by inducing HaCaT cells (control and KD) with TGFβ at different time points. Overview pictures are shown. Nesprin-2, white, was detected with mAb K20–478, SMAD 2/3, red, DAPI staining for nuclei (blue). Arrows point to cells with nuclear accumulation of transcription factor. (B) Numbers of cells showing nuclear localization of SMAD2/3, respectively (n = 500 cells per time point); *p < 0.05). (C-E) Transcript amounts of three different SMADs (SMAD2,3 and 4) in control and KD cells as determined by qRT-PCR.
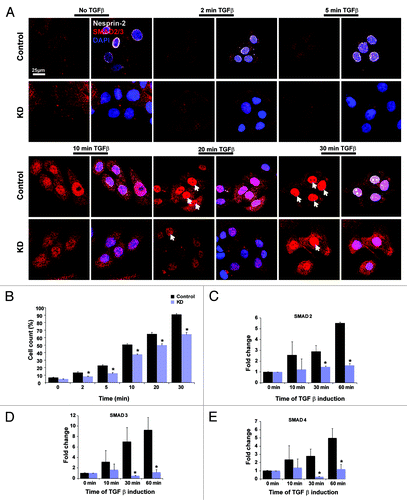
To further confirm our result of reduced expression of SMAD2/3 in Nesprin-2 KD cells, we performed qRT-PCR using RNA isolated from control and KD HaCaT cells at 0, 10, 30 and 60 min after TGFβ stimulation. In WT an increase of SMAD2, 3 and 4 transcripts was seen. In KD cells the levels were significantly reduced and no similar increase over time was detected ().
Nesprin-2 promotes c-Fos translocation to the nucleus
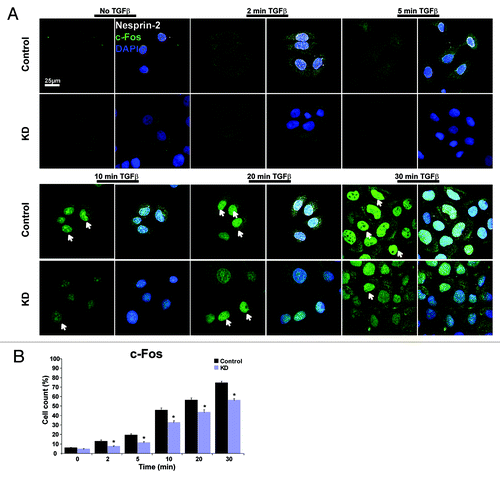
We obtained similar results for c-Fos, a subunit of the AP1 transcription factor. In control cells 10 min after TGFβ stimulation strong nuclear staining was observed whereas KD cells showed a comparable staining only after 20 min (). The analysis of wounded tissue was prevented as c-Fos antibodies did not work reliably for staining of skin sections.
Figure 8. Nesprin-2 mediates the translocation of c-Fos after TGFβ induction. (A) Nuclear translocation of transcription factor c-Fos was studied in control and KD HaCaT cells after induction with TGFβ. Scale bar, 25 µm. (B) Statistical analysis of the percentage of cells showing nuclear localization of c-Fos.
Nesprin-2G associates with heterochromatic DNA
In our analysis we have found that the expression of specific transcription factor genes (PPARβ/δ, Egr-1 and E2F1) is altered during wound healing in Nesprin-2G deficient mice and that the expression and subcellular localization of transcription factor c-Fos and SMAD2, 3 and 4 are affected by Nesprin-2. As we have no indication for a direct interaction of Nesprin-2 and transcription factors, we tested whether Nesprin-2 is involved in chromatin organization and generation of a surrounding for genes which affects their transcription.Citation35,Citation36
We performed chromatin immunoprecipitation (ChIP) with mAb K20-478–4 and control antibodies in HaCaT cells. The precipitated DNA was purified and processed for sequencing. The ChIP-Seq data were analyzed and mapped onto the human genome. We found that the majority of the sequences which could be uniquely mapped to the human genome [March 2006 (NCBI36/hg18) assembly] represented centromere and other heterochromatic sequences derived from almost all chromosomes (~50% centromere and ~26% heterochromatic sequences). Since heterochromatic regions of most chromosomes are underrepresented in the hg18 assembly due to their repetitive nature we think that our mapping approach revealed only a part of all reads assignable to such regions. Significantly fewer reads were derived from coding sequences. Furthermore, the coding sequences were not identical among the independent experiments in general whereas centromere and heterochromatic sequences were consistently observed (). In contrast, the control ChIPs with a GFP antibody or pAbK1 (Nesprin-2 C-terminal antibody) ChIP did not produce this pattern.
Table 1. Results from ChIP Seq experiments performed with HaCaT cells using mAb K20–478 and pAbK1
To get further evidence for a heterochromatin interaction we stained Nesprin-2G deficient and WT fibroblasts with antibodies recognizing heterochromatin protein (HP1β). HP1β and the variant HP1α are predominantly found in foci of constitutive heterochromatin. Staining with antibodies gives a speckled pattern overlaying a diffuse fluorescence but the number of speckles can vary depending on the cell type.Citation37,Citation38 We obtained a speckled pattern in WT and KO primary fibroblasts; however the number of speckles varied. In WT fibroblasts we counted ~10 speckles per nucleus, while in the KO fibroblasts the number was reduced to ~7 speckles. Furthermore, approximately 19% of WT nuclei did not have HP1β speckles. In the KO this number increased to ~36% ( and ). Protein gel blot analysis also showed reduced expression of HP1β in KO cells, tubulin was used as a loading control ().
Figure 9. Nesprin-2 Giant associates with heterochromatic DNA and affects the localization of HP1β. (A) HP1β staining in WT and Nesprin-2 KO fibroblasts. mAb K56–386 was used to recognize Nesprin-2 Giant and DAPI to stain the nucleus (*p < 0.01 and **p < 0.001). (B) protein gel blot analysis showing the expression of HP1β in WT and KO.
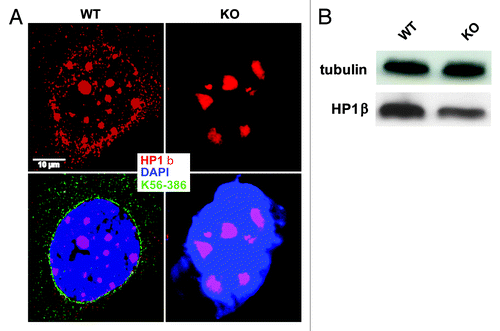
Table 2. Nesprin-2 KO fibroblasts showed reduced heterochromatin protein speckles
HP1β is known to interact with trimethylated histone (H3K9Me3) and this interaction is important to recruit HP1β to the heterochromatin region of the genome.Citation39 We therefore also studied the pattern of H3K9Me3 distribution in Nesprin-2 KD HaCaT cells. In control cells H3K9Me3 was distributed throughout the nucleus and was also seen near the nuclear envelope. Nesprin-2 loss caused an unequal distribution of H3K9Me3 and led to its accumulation in the center of the nucleus (Fig. S4A, indicated by white dotted boxes). These changes in localization were accompanied by reduced expression of the protein (Fig. S4B).
Discussion
The LINC complex forms a connection between the nuclear lamina and cytoskeletal elements of the cytosol. Its importance is highlighted by the findings that mutations in LINC complex components can cause a variety of human diseases called laminopathies. They could arise from adverse effects of the LINC complex on actin mediated cellular functions including cell adhesion, cell migration and cell mechanics or altered gene expression due to altered interactions of transcription factors with Lamin A/C and its associated NE proteins or through changes in chromatin organization.Citation40
The in vivo wound healing experiment in Nesprin2G KO mice allowed us to probe the role of the LINC complex directly in a process that involves directional cell migration, proliferation and differentiation. Remarkably, we observed a delay in wound healing at day 7 and 10 in Nesprin-2G KO mice, which is the phase when keratinocytes and myofibroblasts contribute to the wound healing process. We therefore focused on these two cell types in our analysis. We found changes in the abundance of transcription factors that regulate fibroblast differentiation and keratinocyte proliferation as well as changes in the cytoskeleton which might lead to altered cell mechanics that could also impact on gene regulation. Nesprin-2 KO wounds showed altered keratinocyte differentiation and reduced proliferation and an atypical migration pattern and absence of neo-epidermis at these time points. This could impair the wound sealing from the epidermis. In support, KO as well as KD cells form strong focal adhesions in vitro on a collagen matrix which slows down migration. The increased cell spreading area and focal contacts in KO and KD cells were however not paralleled by increased amounts of Vinculin but might be achieved by its redistribution.
For transcription factors that regulate fibroblast differentiation and keratinocyte proliferation we observed slight alterations in the accumulation of the PPARβ/δ, EGR-1, and E2F1 transcripts in mutant wounded skin. PPARβ/δ and E2F1 expression were approximately 2- and 1.5-fold reduced at day 1 as compared with WT. PPARβ/δ is one of the targets of AP-1 and in the WT situation its expression is strongly enhanced in keratinocytes after skin injury.Citation29 The observed delayed wound healing could be a result of reduced PPARβ/δ expression.Citation41
In wounded skin EGR-1 and E2F1 transcript levels are slightly increased at day 3 in Nesprin-2G KO. E2F1 activity is essential for proper epidermal morphogenesis and keratinocyte proliferation. In differentiating keratinocytes, Ca2+ induced protein kinase C (PKC) activation downregulates E2F1 by activating p38β mitogen-activated protein kinase (MAPK).Citation42 Murine keratinocyte differentiation is associated with loss of E2F1/pRb DNA-binding complexes and E2F1 knock-out mice showed defective keratinocyte proliferation and migration which finally led to delayed wound re-epithelialization.Citation31 This indicates that E2F1 is a crucial regulator of keratinocyte proliferation and migration. Together, a decrease in PPARβ/δ at day 1 and an increase in E2F1 expression at day 3 may cause the slowing down of proliferation in Nesprin-2G KO mice wound healing. It is possible that this sequence of events is caused by a loss of a cellular signal which is normally transduced from the cell surface via cytoskeletal elements to the nucleus through the LINC complex with Nesprin-2 as one of its components or in response to mechanical damage. Once this link is lost due to the absence of Nesprin-2G cell functions such as proliferation and differentiation are delayed, though not completely lost.
A likely signal is TGFβ1. Interestingly, an earlier study had shown that induction of TGFβ1 in fibroblasts enhanced the formation of “structural elements,” namely bundles of actin filaments or stress fibers, vinculin-containing fibronexus adhesion complexes and fibronectin fibrils that caused increased expression of α-SMA.Citation43 These “structural elements” were important for generating contractile force in myofibroblasts which is associated with wound contraction. Formation of stress fibers therefore directly correlates with the expression of α-SMA, and a reduction in α-SMA is equivalent to a reduction in F-actin content. Such a scenario might also be relevant for our findings in the injured skin of Nesprin-2G deficient animals.
In Nesprin-2 KD cells TGFβ1 stimulation led to a retarded nuclear accumulation and expression of SMAD2, 3 and 4 and c-Fos. A direct interaction of these transcription factors with Nesprin-2 could not be shown so far. However, the observed effect could also be achieved through indirect interactions. In such a scenario, Nesprin-2, based on its ability to interact with Emerin and Lamin, can affect TGFβ/Smad signaling as Emerin is directly involved in this signaling pathway through its interaction with MAN1, an important factor of TGFβ/BMP signaling.Citation44 We have previously shown that in the Nesprin-2 Giant KO mouse Emerin is redistributed into the cytoplasm where it can no longer perform its role in these pathways.Citation18
Nesprin-2 might also be directly involved in signaling. In recent work it was shown that a short isoform of Nesprin-2 can act as an extracellular signal-regulated MAPK1 and MAPK2 scaffold protein that plays a role in regulating the nuclear signal.Citation12 This might also be relevant in our wound healing experiments as we have previously shown that loss of Nesprin-2G affected the expression of C-terminal Nesprin-2 isoforms leading to a reduction or their complete loss (see ref. Citation18 and Fig. S1). Another signaling molecule in wound healing is β-catenin. It activates fibroblast proliferation and inhibits keratinocyte migration.Citation45 β-Catenin together with α-Catenin, Emerin and Nesprin-2 forms a complex at the NE in which Nesprin-2 acts as a positive regulator of Wnt signaling, since loss of Nesprin-2 results in a decrease of nuclear β-catenin pools and a downregulation of Wnt pathway activity, one of the signaling pathways β-catenin is involved in.Citation46 β-catenin activation can also be achieved by TGFβ1 and through this link involve Nesprin-2 in mediating downstream events of TGFβ1 signaling.
Interaction of Nesprin-2 with centromeres and heterochromatin within the nucleus suggests a further mechanism through which it might act in transcriptional regulation of genes involved in cell proliferation, namely by bringing the chromatin into a surrounding favorable or unfavorable for activity. It was shown for mouse pericentric heterochromatin that it is involved in proliferation dependent and cell cycle regulated transcription.Citation47-Citation50 Reduction in expression and unequal distribution of HP1β speckles as well as H3K9Me3 in Nesprin-2 KO and KD cells, respectively, show the important role of Nesprin-2 in maintaining the heterochromatin protein pattern. Through this Nesprin-2 could also influence the expression of genes which are naturally residing within the heterochromatin region either as negative or positive regulator.Citation51,Citation52
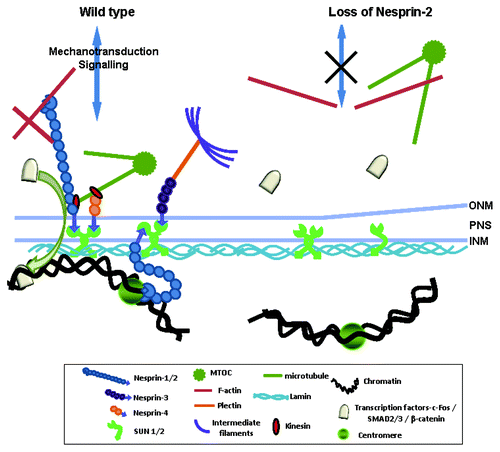
From our study we propose that Nesprin-2G has two major roles in wound healing; one through its capacity as an actin-binding protein and a further one through its interaction with lamina components and with chromatin. At the ONM it interacts with F-actin and is responsible for the correct formation of the actin cytoskeleton around the nucleus in WT. Through the actin cytoskeleton interaction it is also connected to the plasma membrane and the extracellular matrix and can affect focal adhesion formation. At the NE, Nesprin-2 interacts with SUN proteins and the underlying nuclear lamina. Loss of Nesprin-2G leads to a loss of F-actin around the nucleus, and signals from the extracellular matrix are less efficiently transmitted to the NE and the nuclear lamina (). Nuclear lamina components themselves can interact with transcription factors and through this chain of interactions Nesprin-2 may affect transcription. Interestingly, the binding sites of LaminA/C for Rb protein and c-Fos overlap with the binding site for Nesprin-2 and a competition for binding sites might be a further mechanism to explain Nesprin-2 action in addition to its effects on transcription through heterochromatin interactions.Citation7,Citation14 Finally, actin, the interaction partner of Nesprin-2 Giant is also a nuclear protein and has roles in gene regulation.Citation53 Therefore an impact of Nesprin-2 on transcription via actin could also be considered.
Figure 10. Proposed mechanisms of Nesprin-2 involvement in cell migration, proliferation and transcription regulation. In WT Nesprin-2 at the ONM together with other NE proteins connects the nucleus to the actin cytoskeleton (LINC complex). The LINC complex (Nesprin-SUN) and associated proteins can transduce signals from the cytoplasm to the nucleus. Nesprins can transduce either mechanic signals through the LINC complex to the nucleoplasm and are also involved in signal transduction processes through interaction with transcription factors. Gene transcription may also be affected through interactions of NE proteins with chromatin thereby altering the status of chromatin from being inactive or being actively transcribed and vice versa. In KO cells F-actin distribution and status of heterochromatin is altered. Expression and availability of transcription factors is reduced and leads to reduced cell proliferation.
As the changes in the Nesprin-2G KO mice were only observed under physiological stress conditions, there must be other proteins which ensure a correct arrangement of the chromatin and functioning of the cell. Such a mechanism is vital for a cell to survive adverse conditions as during mechanical stress.
Materials and Methods
Nesprin-2G deficient mice
The generation of Nesprin-2G deficient mice has been described.Citation18 Animals were backcrossed into the C57Bl6 background for seven generations. All animals used in the studies were females between 9 and 12 weeks of age; age and sex-matched littermates were used as controls. Animals were housed in specific pathogen-free facilities and all animal protocols were approved by the local veterinary authorities.
Wound healing experiments
Wound healing experiments and analysis were done as described.Citation54 In brief, four circular full-thickness (this is beyond the panniculus carnosus (PC) muscle) excisional wounds of 5 mm in diameter were made on the dorsal side of each mouse. Wounds were harvested at 1, 3, 5, 7 and 10 d after wounding. Six wild type and six knockout animals were used for analysis per time point if not indicated otherwise.
Immunohistochemistry and immunofluorescence
For general histology, the samples (paraffin sections) were stained with hematoxylin and eosin (H&E). For immunofluorescence, paraffin sections were deparaffinised and rehydrated which was then followed by antigen retrieval and antibody incubation.Citation54 Antibodies directed against F4/80 (1:40, CI:A3–1 AbD Serotec), α-SMA (1:500, 1A4, Cy3 conjugate, SIGMA), Ki-67 (1:40, TEC-3, Dako), Keratin 14 (1:1000, AF64, COVANCE), c-Fos (1:1000, sc-52, Santa Cruz Biotechnology), HP1β (1:240, H2039, SIGMA), β-actin (1:1000, A5316, SIGMA), α-tubulin (mAb WA3), Vinculin (1:50, SIGMA), SMAD 2/3 (1:250, #5678, Cell Signaling), H3K9Me3 (1:300, #07442 Upstate) and Nesprin-2G (mAb K20–478,Citation2 mAb K56–386,Citation18 were used. Appropriate secondary antibodies were conjugated with Alexa Fluor 488 and 594 (Molecular Probes). Nuclei were stained with 4, 6-diamino-2-phenylindole (DAPI), F-actin with TRITC-Phalloidin. Cryosections were used for detecting Nesprin-2 with mAb K56–386 in skin sections (Fig. S1A). Cryosections were fixed in acetone for 15 min at -20°C and permeabilized with 0.5% Triton followed by blocking with PBG. Sections were incubated with primary and secondary antibodies for overnight at 4°C and for 1 h at room temperature, respectively. Cultured cells were fixed with paraformaldehyde and processed for immunofluorescence analysis.Citation18
Quantification
Paraffin sections from 5, 7 and 10 d after wounding were stained with α-SMA. To quantify the amount of differentiated fibroblasts fluorescence signal intensity of α-SMA per unit area was measured using “quantify” function in the TCS SP II confocal software.
For measuring the granulation tissue area and distance between the panniculus carnosus, paraffin sections were stained with Sirius red and analyzed with polarized light microscopy.Citation55 For quantification of area and length of hyperproliferative epidermis, Paraffin wound sections were stained with H&E. These sections (n = 3–6 sections/wound/mouse) of indicated time points were analyzed using DISKUS software. To study nuclear localization of c-Fos and SMAD2/3, HaCaT cells were transfected with control and KD plasmids. After treating with TGFβ (1 ng/ml) (Cell Signaling Technology #5231), cells were subjected to immunofluorescence with indicated antibodies. For each time point, 500 cells were counted randomly and out of them cells showing nuclear localization of c-Fos and SMAD2/3 were counted and then their percentage calculated.
RNA isolation and quantitative Real Time PCR (qRT-PCR)
Wounds were excised and collected at 1, 3, 5, 7 and 10 d after wounding and immediately frozen in liquid nitrogen. Tissues were homogenized with an ULTRA TURRAX (IKA LABORTECHNIK) tissue homogenizer and RNA was isolated using TRIZOL (Invitrogen). Quantity and quality of RNA was analyzed on an Agilent Bioanalyser (Agilent Technologies). cDNA was prepared by reverse transcription of 5 μg RNA with oligo dTCitation18 using Superscript II reverse transcriptase (Invitrogen). Real time PCR was performed with the Opticon III instrument (MJ Research) using the Quantitect™ SYBR® green PCR kit (Qiagen) according to Farbrother et al.Citation56 As a quantification standard defined concentrations of annexinA7 cDNACitation57 were used for amplification. For every cDNA quantification three reactions were performed in parallel per animal, quantification results were normalized based on the GAPDH control and mean values were calculated.
Cell culture and cell scratch assay
Dermal fibroblasts and keratinocytes were isolated from new born mice of both WT and Nesprin-2 KO. Dermal fibroblasts (passage 2 and 3; three mice each) were cultured in DMEM medium with 10% fetal bovine serum (FBS), L-Glutamine and antibiotics like Penicillin-Streptomycin in 5% CO2 and 37°C incubator. Whereas primary keratinocytes were cultured under 5% CO2 and 32°C incubator in low Ca2+ FAD medium on type I collagen coated plates on which a feeder layer of mitomycin-treated 3T3 fibroblasts had been prepared. Low Ca2+ FAD medium was supplemented with 10% fetal calf serum (FCS) treated with Chelex 100 resin (BioRad) to reduce the calcium concentration and a cocktail of 1.8 × 10–10 M adenine, hydrocortisone (0.5 μg/ml), insulin (5 μg/ml), cholera toxin (10–10 M) and epidermal growth factor (EGF) (10 ng/ml).Citation58,Citation59 Migration of wild type and mutant keratinocytes after scratching was analyzed by time lapse video microscopy (32°C, 5% CO2) using a Leica DMIRE2 microscope.
Expression of Nesprin-2 was depleted from HaCaT keratinocytes using plasmid based shRNA as described.Citation18 For Nesprin-2 knock down Nesprin-2 C-terminal sequences were cloned into the pSHAG-1 vector using BseRI and BamHI restriction sites.Citation60
Nesprin-2 C-terminal (N2-C2) - 5′C CCAGCCTCCTGCAACATCCGAAGCTTGGGATGTTGCAGGAGGCTGGTTTTTT; Nesprin-2 C-terminal (N2-C2) - 3′ GATCCCAGCCTCCTGCAACATCCCAAGCTTCGGATGTTGCAGGAGGCTGGCG; and Control- 5′ ATCTACTCGACGTGAGCGTGAAGCTTGACGCTCACGTCGAGTAGATTTTTT; and Control- 3′ GATCAAAAAATCTACTCGACGTGAGCGTCAAGCTTCACGCTCACGTCGAGTAGATCG. HaCaT cells were transfected twice at intervals of 3 d using the Amaxa cell line Nucleofector® kit V (Lonza) according to the manufacturer's instructions. For TGFβ signaling cells were treated with 1ng/ml TGFβ for different times (5, 10, 20 and 30 min) and used for analysis.
Time lapse video microscopy
For cell scratch assays, mouse primary keratinocytes from Nesprin-2 KO and WT were subjected to live cell imaging using Leica DM IRE 2 microscope and Leica DFC 350 FX camera with Leica FW 4000 software. Images were taken every 10 min up to 48 h. Images taken at 0 and 48 h time points were used for quantification of cell migration speed. The distance between the migrating cells at different positions was measured using ImageJ tool.
Chromatin immunoprecipitation and DNA sequencing
Chromatin immunoprecipitation (ChIP) was performed in HaCaT cells using a kit (ChIP-IT Express, 53008, Active Motif). The protocol of the manufacturer was essentially followed. Sequencing methods were based on Illumina protocols (Illumina Inc). mAb K20–478 was used for precipitation of Nesprin-2 Giant.Citation2 In control ChIP experiments pAbK1 recognizing C-terminal isoforms of Nesprin-2 and a GFP-specific antibody were used.Citation7
Additional material
Download Zip (3.7 MB)Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by the Center for Molecular Medicine Cologne (CMMC) and the Cologne Excellence Cluster on Cellular Stress Responses in Aging-Associated Diseases (CECAD). We thank Dr Ralf Hallinger and Profs. Carien Niessen and Sabine Eming (Department of Dermatology, University Hospital Cologne) for helpful suggestions, Dr. U. Euteneuer for providing WA3 antibody, and Rolf Müller, Berthold Gaßen and Martina Munck for help throughout the course of the experiments.
References
- Zhang Q, Skepper JN, Yang F, Davies JD, Hegyi L, Roberts RG, et al. Nesprins: a novel family of spectrin-repeat containing proteins that localize to the nuclear membrane in multiple tissues. J Cell Sci 2001; 114:4485 - 98; PMID: 11792814
- Zhen YY, Libotte T, Munck M, Noegel AA, Korenbaum E. NUANCE a giant protein connecting the nucleus and actin cytoskeleton. J Cell Sci 2002; 115:3207 - 22; PMID: 12118075
- Padmakumar VC, Abraham S, Braune S, Noegel AA, Tunggal B, Karakesisoglou I, et al. Enaptin a giant actin-binding protein, is an element of the nuclear membrane and the actin cytoskeleton. Exp Cell Res 2004; 295:330 - 9; http://dx.doi.org/10.1016/j.yexcr.2004.01.014; PMID: 15093733
- Wilhelmsen K, Litjens SH, Kuikman I, Tshimbalanga N, Janssen H, Van den Bout I, et al. Nesprin-3, a novel outer nuclear membrane protein, associates with the cytoskeletal linker protein plectin. J Cell Biol 2005; 171:799 - 810; http://dx.doi.org/10.1083/jcb.200506083; PMID: 16330710
- Roux KJ, Crisp ML, Liu Q, Kim D, Kozlov S, Stewart CL, et al. Nesprin4 is an outer nuclear membrane protein that can induce kinesin mediated cell polarization. Proc Natl Acad Sci USA 2009; 106:2194 - 9; http://dx.doi.org/10.1073/pnas.0808602106; PMID: 19164528
- Simpson JG, Roberts RG. Patterns of evolutionary conservation in the nesprin genes highlight probable functionally important protein domains and isoforms. Biochem Soc Trans 2008; 36:1359 - 67; http://dx.doi.org/10.1042/BST0361359; PMID: 19021556
- Libotte T, Zaim H, Abraham S, Padmakumar VC, Schneider M, Lu W, et al. LaminA/C-dependent localization of Nesprin-2, a giant scaffolder at the nuclear envelope. Mol Biol Cell 2005; 16:3411 - 24; http://dx.doi.org/10.1091/mbc.E04-11-1009; PMID: 15843432
- Starr DA, Fridolfsson HN. Interactions between nuclei and the cytoskeleton are mediated by SUN-KASH nuclear-envelope bridges. Annu Rev Cell Dev Biol 2010; 26:421 - 44; http://dx.doi.org/10.1146/annurev-cellbio-100109-104037; PMID: 20507227
- Andres V, Gonzalez JM. Role of A-type lamins in signaling, transcription, and chromatin organization. J Cell Biol 2009; 187:945 - 57; http://dx.doi.org/10.1083/jcb.200904124; PMID: 20038676
- Pare GC, Easlick JL, Mislow JM, McNally EM, Kapiloff MS. Nesprin-1 alpha contributes to the targeting of mAKAP to the cardiac myocyte nuclear envelope. Exp Cell Res 2005; 303:388 - 99; http://dx.doi.org/10.1016/j.yexcr.2004.10.009; PMID: 15652351
- Mislow JM, Holaska JM, Kim MS, Lee KK, Segura-Totten M, Wilson KL, et al. Nesprin-alpha self-associated and binds directly to emerin and lamin A in vitro. FEBS Lett 2002; 525:135 - 40; http://dx.doi.org/10.1016/S0014-5793(02)03105-8; PMID: 12163176
- Warren DT, Tajsic T, Mellad JA, Searles R, Zhang Q, Shanahan CM. Novel nuclear nesprin-2 variant tether active extracellular signal-regulate MAPK1 and MAPK2 at promyelocytic leukemia protein nuclear bodies and act to regulate smooth muscle cell proliferation. J Biol Chem 2010; 285:1311 - 20; http://dx.doi.org/10.1074/jbc.M109.032557; PMID: 19861416
- Schneider M, Lu W, Neumann S, Brachner A, Gotzmann J, Noegel AA, et al. Molecular mechanisms of centrosome and cytoskeleton anchorage at the nuclear envelope. Cell Mol Life Sci 2011; 68:1593 - 610; http://dx.doi.org/10.1007/s00018-010-0535-z; PMID: 20922455
- Maraldi NM, Lattanzi G, Cenni V, Bavelloni A, Marmiroli S, Manzoli FA. Laminopathies and lamin-associated signalling pathways. J Cell Biochem 2011; 112:979 - 92; http://dx.doi.org/10.1002/jcb.22992
- Verstraeten VL, Broers JL, Ramaekers FC, Van Steensel MA. The nuclear envelope, a key structure in celluar integrity and gene expression. Curr Med Chem 2007; 14:1231 - 48; http://dx.doi.org/10.2174/092986707780598032; PMID: 17504143
- Puckelwartz MJ, Kessler E, Zhang Y, Hodzic D, Randles KN, Morris G, et al. Disruption of nesprin-1 produces an Emery Dreifuss muscular dystrophy-like phenotype in mice. Hum Mol Genet 2009; 18:607 - 20; http://dx.doi.org/10.1093/hmg/ddn386; PMID: 19008300
- Zhang Q, Bethmann C, Worth NF, Davies JD, Wasner C, Feuer A, et al. Nesprin-1 and-2 are involved in the pathogenesis of Emery Dreifuss muscular dystrophy and are critical for nuclear envelope integrity. Hum Mol Genet 2007; 16:2816 - 33; http://dx.doi.org/10.1093/hmg/ddm238; PMID: 17761684
- Lüke Y, Zaim H, Karakesisoglou I, Jaeger VM, Sellin L, Lu W, et al. Nesprin-2 Giant (NUANCE) maintains nuclear envelope architecture and composition in skin. J Cell Sci 2008; 121:1887 - 98; http://dx.doi.org/10.1242/jcs.019075; PMID: 18477613
- Zhang X, Xu R, Zhu B, Yang X, Ding X, Duan S, et al. Syne-1 and Syne-2 play crucial roles myonuclear anchorage and motor neuron innvervation. Development 2007; 134:901 - 8; http://dx.doi.org/10.1242/dev.02783; PMID: 17267447
- Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol 2007; 127:526 - 37; http://dx.doi.org/10.1038/sj.jid.5700613; PMID: 17299435
- Blumbach K, Zweers MC, Brunner G, Peters AS, Schmitz M, Schulz JN, et al. Defective granulation tissue formation in mice with specific ablation of integrin-linked kinase in fibroblasts - role of TGFbeta1 levels and RhoA activity. J Cell Sci 2010; 123:3872 - 83; http://dx.doi.org/10.1242/jcs.063024; PMID: 20980390
- Aksoy MH, Vargel I, Canter IH, Erk Y, Sargon M, Pinar A, et al. A new experimental hypertrophic scar model in guinea pigs. Aesthetic Plast Surg 2002; 26:388 - 96; http://dx.doi.org/10.1007/s00266-002-1121-z; PMID: 12432481
- Chancellor TJ, Lee J, Thodeti CK, Lele T. Actomyosin Tension Exerted on the Nucleus through Nesprin-1 Connections Influences Endothelial Cell Adhesion, Migration and Cyclic Strain-Induced Reorientation. Biophys J 2010; 99:115 - 23; http://dx.doi.org/10.1016/j.bpj.2010.04.011; PMID: 20655839
- Khatau SB, Hale CM, Stewart-Hutchinson PJ, Patel MS, Stewart CL, Searson PC. etz al. A perinuclear actin cap regulates nuclear shape. Proc Natl Acad Sci USA 2009; 106:19017 - 22; http://dx.doi.org/10.1073/pnas.0908686106; PMID: 19850871
- Reddy KL, Zullo JM, Bertolino E, Singh H. Transcriptional repression mediated by repositioning of genes to the nuclear lamina. Nature 2008; 452:243 - 7; http://dx.doi.org/10.1038/nature06727; PMID: 18272965
- Taniura H, Glass C, Gerace L. A chromatin binding site in the tail domain of nuclear lamins that interacts with core histones. J Cell Biol 1995; 131:33 - 44; http://dx.doi.org/10.1083/jcb.131.1.33; PMID: 7559784
- Dorner D, Vlcek S, Foeger N, Gajewski A, Makolm C, Gotzmann J, et al. Lamina-associated polypeptide 2alpha regulates cell cycle progression and differentiation via the retinoblastoma-E2F pathway. J Cell Biol 2006; 173:83 - 93; http://dx.doi.org/10.1083/jcb.200511149; PMID: 16606692
- Heessen S, Fornerod M. The inner nuclear envelope as a transcription factor resting place. EMBO Rep 2007; 8:914 - 9; http://dx.doi.org/10.1038/sj.embor.7401075; PMID: 17906672
- Michalik L, Desvergne B, Tan NS, Basu-Modak S, Escher P, Rieusset J, et al. Impaired skin wound healing in peroxisome proliferator-activated receptor (PPAR) alpha and PPAR beta mutant mice. J Cell Biol 2001; 154:799 - 814; http://dx.doi.org/10.1083/jcb.200011148; PMID: 11514592
- Tan NS, Michalik L, Noy N, Yasmin R, Pacot C, Heim M, et al. Critical roles of PPAR beta/delta in keratinocyte response to inflammation. Genes Dev 2001; 15:3263 - 77; http://dx.doi.org/10.1101/gad.207501; PMID: 11751632
- D’Souza SJ, Vespa A, Mukherjee S, Maher A, Pajak A, Dagnino L. E2F-1 is essential for normal epidermal wound repair. J Biol Chem 2002; 277:10626 - 32; http://dx.doi.org/10.1074/jbc.M111956200; PMID: 11790795
- Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev 2003; 83:835 - 70; PMID: 12843410
- Batut J, Howell M, Hill CS. Kinesin-mediated transport of Smad2 is required for signaling in response to TGF-beta ligands. Dev Cell 2007; 12:261 - 74; http://dx.doi.org/10.1016/j.devcel.2007.01.010; PMID: 17276343
- Rahimi RA, Leof EB. TGF-beta signaling: a tale of two responses. J Cell Biochem 2007; 102:593 - 608; http://dx.doi.org/10.1002/jcb.21501; PMID: 17729308
- Ivorra C, Kubicek M, Gonzalez JM, Sanz-Gonzalez SM, Alvarez-Barrientos A, O’Connor JE, et al. A mechanism of AP-1 suppression through interaction of c-Fos with lamin A/C. Genes Dev 2006; 20:307 - 20; http://dx.doi.org/10.1101/gad.349506; PMID: 16452503
- Gonzalez JM, Navarro-Puche A, Casar B, Crespo P, Andres V. Fast regulation of AP-1 activity through interaction of lamin A/C, ERK1/2 and c-Fos at the nuclear envelope. J Cell Biol 2008; 183:653 - 66; http://dx.doi.org/10.1083/jcb.200805049; PMID: 19015316
- Dialynas GK, Terjung S, Brown JP, Aucott RL, Baron-Luhr B, Singh PB, et al. Plasticity of HP1 proteins in mammalian cells. J Cell Sci 2007; 120:3415 - 24; http://dx.doi.org/10.1242/jcs.012914; PMID: 17855382
- Dialynas GK, Makatsori D, Kourmouli N, Theodoropoulos PA, McLean K, Terjung S, et al. Methylation-independent binding to histone H3 and cell cycle-dependent incorporation of HP1 beta into heterochromatin. J Biol Chem 2006; 281:14350 - 60; http://dx.doi.org/10.1074/jbc.M600558200; PMID: 16547356
- Fischle W, Wang Y, Jacobs SA, Kim Y, Allis CD, Khorasanizadeh S. Molecular basis for the discrimination of repressive methyl-lysine marks in histone H3 by Polycomb and HP1 chromodomains. Genes Dev 2003; 17:1870 - 81; http://dx.doi.org/10.1101/gad.1110503; PMID: 12897054
- Hale CM, Shrestha AL, Khatau SB, Stewart-Hutchinson PJ, Hernandez L, Stewart CL, et al. Dysfunctional connections between the nucleus and the actin and microtubule networks in laminopathic models. Biophys J 2008; 95:5462 - 75; http://dx.doi.org/10.1529/biophysj.108.139428; PMID: 18790843
- Neub A, Houdek P, Ohnemus U, Moll I, Brandner JM. Biphasic regualation of AP-1 subunits during human epidermal wound healing. J Invest Dermatol 2007; 127:2453 - 62; http://dx.doi.org/10.1038/sj.jid.5700864; PMID: 17495958
- Ivanova IA, D’Souza SJ, Dagnino L. E2F1 stability is regulated by a novel-PKC/p38beta MAP kinase signalling pathway during keratinocyte differentiation. Oncogene 2006; 25:430 - 7; PMID: 16116476
- Singer II, Kawka DW, Kazazis DM, Clark RA. In Vivo co-distribution of fibronectin and actin fibers in granulation tissue: immunofluorescence and electron microscope studies of the fibronexus at the myofibroblast surface. J Cell Biol 1984; 98:2091 - 106; http://dx.doi.org/10.1083/jcb.98.6.2091; PMID: 6373789
- Bengtsson L. What MAN1 does to the Smads. TGFbeta/BMP signaling and the nuclear envelope. FEBS J 2007; 274:1374 - 82; http://dx.doi.org/10.1111/j.1742-4658.2007.05696.x; PMID: 17489095
- Cheon SS, Wei Q, Gurung A, Youn A, Bright T, Poon R, et al. Beta-catenin regulates wound size and mediates the effect of TGF-beta in cutaneous healing. FASEB J 2006; 20:692 - 701; http://dx.doi.org/10.1096/fj.05-4759com; PMID: 16581977
- Neumann S, Schneider M, Daugherty RL, Gottardi CJ, Eming SA, Beijer A, et al. Nesprin-2 interacts with alpha-catenin and regulates Wnt signaling at the nuclear envelope. J Biol Chem 2010; 285:34932 - 8; http://dx.doi.org/10.1074/jbc.M110.119651; PMID: 20801886
- Yang L, Peng R. Unveiling hair follicle stems cells. Stem Cell Rev 2010; 6:658 - 64; http://dx.doi.org/10.1007/s12015-010-9172-z; PMID: 20676942
- Lu J, Gilbert DM. Proliferation-dependent and cell cycle regulated transcription of mouse pericentric heterochromatin. J Cell Biol 2007; 179:411 - 21; http://dx.doi.org/10.1083/jcb.200706176; PMID: 17984319
- De Koning L, Savignoni A, Boumendil C, Rehman H, Asselain B, Sastre-Garau X, et al. Heterochromatin protein 1 alpha: a hallmark of cell proliferation relevant to clinical oncology. EMBO Mol Med 2009; 1:178 - 91; http://dx.doi.org/10.1002/emmm.200900022; PMID: 20049717
- Vakoc CR, Mandat SA, Olenchock BA, Blobel GA. Histone H3 lysine9 methylation and HP1 gamma are associated with transcription elongation through mammalian chromatin. Mol Cell 2005; 19:381 - 91; http://dx.doi.org/10.1016/j.molcel.2005.06.011; PMID: 16061184
- Deniaud E, Bickmore WA. Transcription and the nuclear periphery: edge of darkness?. Curr Opin Genet Dev 2009; 19:187 - 91; http://dx.doi.org/10.1016/j.gde.2009.01.005; PMID: 19231154
- De Lucia F, Ni JQ, Vaillant C, Sun FL. HP1 modulates the transcription of cell-cycle regulators in Drosophila melanogaster. Nucleic Acids Res 2005; 33:2852 - 8; http://dx.doi.org/10.1093/nar/gki584; PMID: 15905474
- Schleicher M, Jockusch BM. Actin: its cumbersome pilgrimage through cellular compartments. Histochem Cell Biol 2008; 129:695 - 704; http://dx.doi.org/10.1007/s00418-008-0430-y; PMID: 18438682
- Zweers MC, Davidson JM, Pozzi A, Hallinger R, Janz K, Quondamatteo F, et al. Integrin alpha2beta is required for regulation of murine wound angiogenesis but is dispensable for reepithelialisation. J Invest Dermatol 2007; 127:467 - 78; http://dx.doi.org/10.1038/sj.jid.5700546; PMID: 16977325
- Cryderman DE, Grade SK, Li Y, Fanti L, Pimpinelli S, Wallrath LL. Role of Drosophila HP1 in euchromatic gene expression. Dev Dyn 2005; 232:767 - 74; http://dx.doi.org/10.1002/dvdy.20310; PMID: 15704177
- Farbrother P, Wagner C, Na J, Tunggal B, Morio T, Urushihara H, et al. Dictyostelium transcriptional host cell response upon infection with Legionella. Cell Microbiol 2006; 8:438 - 56; http://dx.doi.org/10.1111/j.1462-5822.2005.00633.x; PMID: 16469056
- Döring V, Veretout F, Albrecht R, Mühlbauer B, Schlatterer C, Schleicher M, et al. The in vivo role of annexin VII (synexin): characterization of an annexin VII-deficient Dictyostelium mutant indicates an involvement in Ca(2+)-regulated processes. J Cell Sci 1995; 108:2065 - 76; PMID: 7657724
- Grimm PC, Nickerson P, Gough J, McKenna R, Stern E, Jeffery J, et al. Computerized image analysis of Sirius Red-stained renal allograft biopsies as a surrogate marker to predict long-term allograft function. J Am Soc Nephrol 2003; 14:1662 - 8; http://dx.doi.org/10.1097/01.ASN.0000066143.02832.5E; PMID: 12761269
- Tscharntke M, Pofahl R, Chrostek-Grashoff A, Smyth N, Niessen C, Niemann C, et al. Impaired epidermal wound healing in vivo upon inhibition or deletion of Rac1. J Cell Sci 2007; 120:1480 - 90; http://dx.doi.org/10.1242/jcs.03426; PMID: 17389689
- Paddison PJ, Caudy AA, Bernstein E, Hannon GJ, Conklin DS. Short hairpin RNAs (shRNAs) induce sequence-specific silencing in mammalian cells. Genes Dev 2002; 16:948 - 58; http://dx.doi.org/10.1101/gad.981002; PMID: 11959843