Abstract
The spatial folding of chromatin has been proposed to be involved in the regulation and coordination of gene expression. The mammalian Hox gene clusters form a particularly interesting case of coordinated gene regulation. Within each Hox cluster, the linear order of the genes closely reflects their temporal and anatomical expression pattern. This striking phenomenon suggests that the overall structure of the Hox clusters is important for their regulation. Recent studies employing chromatin conformation capture techniques indicate that Hox clusters adopt a remarkable spatial configuration, in which active and inactive genes are segregated into two distinct chromatin compartments. Here we discuss the possible underlying mechanisms and regulatory roles of this spatial compartmentalization.
Introduction
The 3D organization of chromosomes is believed to facilitate interactions among genes and their regulatory elements. For example, an enhancer can be located tens or hundreds of kilobases away from the promoter that it regulates, and yet somehow the topology of the intervening DNA segment allows the enhancer and promoter to physically contact each other.Citation1 At a wider scale, it has been proposed that multiple genes may be brought together in the same nuclear compartment, as a means to coordinate their expression states.Citation2
With the development of the chromosome conformation capture (3C) technique,Citation3 researchers have gained a tool to analyze such interactions at the molecular level. 3C determines the relative frequency of direct physical contact between two given genomic loci in a population of cells. Subsequently, more powerful modifications of the approach, such as 4C, 5C, 6C and Hi-C, were developed that allow for the analysis of large numbers of interactions in parallel.Citation4-Citation8 Briefly, 4C and its variants can identify genome-wide interactions of one selected locus, whereas 5C and Hi-C are suitable for the monitoring of interactions of respectively multiple selected or all loci.Citation9
The first usage of 4C revealed an abundance of long-range chromatin interactions, most of which are intrachromosomal. In addition, active loci interact preferentially with other active loci, and inactive loci primarily interact with inactive ones.Citation5 This general trend was later documented at the genome-wide level by using Hi-C.Citation8 Thus, there appears to be an overall spatial separation of active and inactive chromatin in the nucleus. The active genes may cluster in so-called transcription factories located in the nuclear interior,Citation10 whereas the transcriptionally inactive chromatin compartments might correspond in part to nuclear lamina- and nucleolus-associated domains (LADs and NADs, respectively).Citation11,Citation12
The interaction patterns of housekeeping genes (genes that are expressed in all cell types) seem to be rather conserved between different tissues. In contrast, the spatial genomic environments of tissue-specific genes are more variable, indicating that chromosome architecture is quite plastic and linked to the regulation of gene expression.Citation5 A striking example of this are the mammalian Hox gene clusters, of which the 3D organization was recently studied in detail.Citation13-Citation15
Structure, Expression Pattern and Epigenetic Marks of Mammalian Hox Clusters
The evolutionarily conserved Hox genes play crucial roles in the developmental program of all animals. In vertebrates, they specify positional identity along the anterior-posterior axis of the developing vertebral column and neural tube, and along the proximodistal axis of developing limbs. In mammals, there are 39 Hox genes that are organized in four clusters, HoxA, HoxB, HoxC and HoxD, of 13 paralogue groups. The clusters are located on different chromosomes and in each cluster all the genes are oriented in the same direction (5′→3′) (). In general, the genes from the same paralogous group exhibit similar expression patterns and perform redundant functions. Interestingly, there is a remarkable correlation between the location of a particular Hox gene within a cluster and its temporal and spatial expression during embryonic trunk development that results in a series of overlapping gene expression patterns. This phenomenon is referred to as colinearity.Citation18 Thus, the genes located more 3′ in each cluster are activated earlier in development and display a more anterior boundary of expression than those located more 5′ in the cluster. Some Hox genes also display defined posterior expression boundaries, while others are expressed up to the most posterior end of the developing embryoCitation16 (). In developing limb buds, the expression patterns of the HoxA and HoxD genes essentially follow the same colinearity rule: 3′ genes are expressed earlier and more proximally and 5′ genes later and more distally.Citation16
Figure 1. A simplified scheme of the cluster organization and embryonic expression patterns of mammalian Hox genes, exemplified by murine ones. (A) The Hox genes are organized in four clusters, HoxA-D, located on different chromosomes (adapted from ref. Citation16). Paralogous genes are shown in the same color. (B) The mRNA expression patterns of representative HoxD genes along the anterior–posterior axis of the developing mouse embryo. None of the HoxD genes is expressed in forebrain. Source: EMAGE gene expression database.Citation17
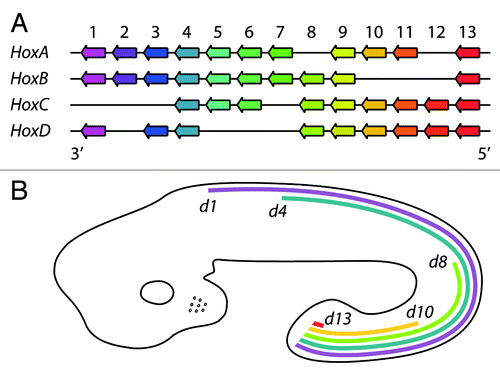
The expression patterns of the Hox genes in some tissues of the adult organism resemble those observed during embyonic development. Profiling of mRNA in human fibroblasts obtained from different anatomic sites identified Hox gene expression signatures that demarcate the anterior and posterior halves of the body and proximal and distal compartments of limbs. For example, fibroblasts isolated from lung express only 3′ HoxA genes and fibroblasts from foot express only 5′ HoxA genes.Citation13,Citation19
The molecular mechanisms that determine these specific temporal and spatial expression patterns of the Hox genes are still poorly understood. Nevertheless, some key regulatory proteins have been identified. Polycomb group (PcG) proteins are responsible for the maintenance of the transcriptionally repressed state of the Hox genes. These proteins form at least two functionally distinct complexes: PRC1 and PRC2. The components of PRC2 trimethylate H3K27 that assists in the recruitment of PRC1. This way, broad domains of H3K27me3 mark the inactive genes in the Hox clusters.Citation20 A transcriptionally active state of the Hox genes is maintained by MLL/Trithorax group (TrxG) proteins and marked by H3K4me2, H3K4me3, and H3ac (H3K9ac/H3K14ac).Citation20 It was recently demonstrated that the successive transcriptional activation of the Hox genes in the developing mouse embryo is intimately linked to the loss of the repressive H3K27me3 mark and acquisition of the active marks.Citation21 As we will discuss below, evidence is accumulating that the spatial organization of chromatin might also be involved in the regulation of the Hox genes expression.
Dynamic 3D Architecture of Mammalian Hox Clusters
That the spatial organization of Hox genes is dynamically linked to their expression pattern was initially revealed by fluorescent in situ hybridization (FISH) microscopy studies.Citation22,Citation23 Induction of differentiation of mouse embryonic stem cells (ESCs) by retinoic acid leads to decondensation of the HoxB cluster that is detectable by FISH, suggesting a dramatic reshaping of the cluster. This decondensation is followed by looping of activated genes outside the chromosome territory (CT). Four days after the initiation of differentiation, both the active Hoxb1 and inactive Hoxb9 genes are marked by histone H3K4me2 and H3K9Ac, but only the Hoxb1 gene is located outside the CT. The Hoxb9 gene leaves the CT about six days later, which correlates well with its transcriptional activation.Citation22 Similar results were obtained in the developing mouse embryo.Citation23 These results point to a remarkable spatial reorganization of the cluster upon Hox gene activation.
Several studies have begun to unravel the 3D architecture of the Hox clusters at much higher resolution, using 3C and derivative techniques. First, 4C analysis revealed an increase of inter-chromosomal interactions for the Hoxb1 gene during differentiation of mouse ESCs,Citation6 in agreement with the extrusion of the gene from the CT. Next, 3C and 5C studies in human cancer cells found extensive long-range contacts within all Hox clusters,Citation24,Citation25 but it was difficult to deduce general patterns from these data sets, perhaps due to the tumor origin of the cells. Much clearer pictures emerged from recent comprehensive 4C and 5C studies in developing mouse embryo and human primary fibroblasts.Citation13,Citation14 Direct comparisons of cells or tissues originating from different anatomical positions revealed intriguing correlations between transcription patterns and chromatin organization of the Hox clusters. In each case, the transcriptionally active portions of Hox clusters appear to form a single spatial compartment, within which a multitude of short-range and long-range contacts occur. This entire compartment exhibits very few contacts with the transcriptionally silent segment of the cluster.Citation13,Citation14 Rather, this inactive portion forms a spatially separate compartment. Somewhat puzzlingly, the strength of the interactions among the silent loci is much less pronounced in human primary fibroblasts than in mouse embryonic cells.Citation13 This may be due to species- or tissue-specificity or due to the methods used.
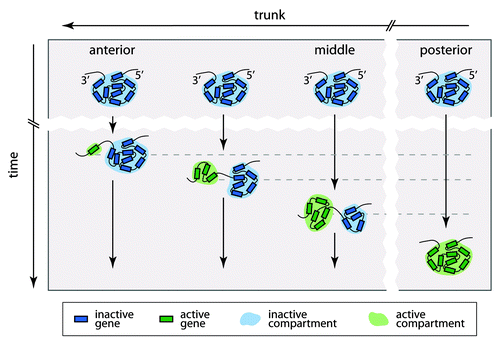
Importantly, the spatial organization of the Hox clusters is dynamic during development, and there is a remarkable coincidence between the transcriptional activity of a gene and its association with the active or repressed spatial compartment. For example, in developing mouse forebrain, all Hox genes are completely silent and each entire cluster forms only a single spatial compartment.Citation14 Toward the posterior end of the trunk, an increasing number of neighboring Hox genes relocates from the inactive into the active spatial compartment (). An exception is an 80 kb repeat-rich intergenic region between the Hoxb9 and Hoxb13 genes, which interacts with neither the active nor the inactive compartment, suggesting that it forms a spatially distinct unit.Citation14 Considered together, the results argue that the bipartite 3D architecture of the Hox clusters is dynamic and closely linked to the colinear expression of the genes during embryogenesis.
Figure 2. A model of the spatial reorganization of a Hox cluster in the mouse trunk during embryonic development. At the early stages of embryogenesis, the Hox cluster is inactive and forms a single spatial compartment in all cells along the trunk. Later on, the Hox genes become sequentially activated according to the colinearity rules and form a separate spatial compartment. This results in the bipartite spatial organization of the cluster, with an increasingly expanded active compartment toward the posterior end of the trunk. It is likely that in the most posterior part of the trunk, all genes of some clusters become activated and form a single spatial compartment.
Mechanisms that Determine the 3D Architecture of Hox Clusters
The spatial separation of the active and inactive segments of the Hox clusters shows a tight correlation with the domain-like deposition patterns of H3K4me3 and H3K27me3, respectively. These marks define two different chromatin “states” that each harbor a variety of specific proteins that could help to set up the 3D architecture. Interestingly, chromatin marked by H3K27me3 and bound by PcG proteins is known to have a tendency to aggregate, both in vitro and in vivo.Citation7,Citation26 Indeed, the higher-order chromatin compaction of the silent HoxB cluster in mouse ESCs requires the Ring1B protein, a component of PRC1, in addition to H3K27me3. Heterozygous deletion of the Ring1B gene leads to partial decompaction of the HoxB and HoxD clusters without detectable increase in gene expression, strongly suggesting that decompaction is not simply the consequence of transcriptional activity.Citation27 In part, therefore, PcG proteins may “glue” the inactive genes in each Hox cluster together into a distinct compartment.
Yet, this hypothesis does not fully explain the bipartite spatial configuration of the Hox clusters. In fact, the active genes in each Hox cluster show a stronger tendency to interact among themselves than the inactive genes, at least in human fibroblasts.Citation13 It is therefore likely that there are also mechanisms promoting the aggregation of the active genes into a separate compartment. A wide range of proteins, such as CTCF, cohesin, SATB1 and C-MYC, have been proposed to have the cohering activities.Citation28 Although in the case of Hox genes their roles need to be determined.
A range of regulatory elements within and next to the Hox clusters might be responsible for the domain organization (both spatially and in terms of histone marks).Citation29 A remarkable example is a ~600 kb “archipelago” of regulatory “islands” that was recently discovered in the large gene desert proximal to the 5′ end of the mouse HoxD cluster.Citation15 Genetic analysis demonstrated that multiple elements in this region contribute to the robust expression of the Hoxd10–13 genes in developing digits. Genetic analysis demonstrated that multiple elements in this region contribute to the robust expression of the Hoxd10-13 genes in developing digits, since deletion of these elements leads to malformations of the digits. Interestingly, 4C studies revealed that these novel regulatory elements strongly interact with the Hoxd10–13 region and much less with the inactive Hoxd1–8 region. Thus, in developing digits, the transcriptionally active 3D compartment is much bigger and includes multiple elements from the 600 kb “archipelago” region. Similarly, the inactive compartment appears to be in physical contact with another large gene desert region distal to the 3′ end of the cluster.Citation15 Perhaps this latter region harbors a series of elements that promote the repressed state, although this hypothesis requires further testing.
Another interesting class of candidate “regulatory elements” are the many long intergenic noncoding (linc) RNA transcription units that reside in the Hox clusters.Citation30 One of these is the HOTAIR lincRNA gene that is located in the HoxC locus between Hoxc11 and Hoxc12 genes. Its expression pattern follows the colinearity rule: the lincRNA is detected predominantly in anatomically posterior and distal tissues. The HOTAIR lincRNA interacts biochemically and functionally with the PRC2 complex.Citation30 Surprisingly, knockdown of HOTAIR does not affect any gene in the HoxC cluster, but leads to derepression of genes in the HoxD locus. This effect may be specific for human cells, as deletion of mHotair (as part of a larger deletion) in mouse did not have any detectable effect.Citation31
Another lincRNA named HOTTIP might be involved in the deposition of H3K4me3 within the human HoxA cluster.Citation13 The HOTTIP gene is located 5′ to the Hoxa13 and its expression pattern is also governed by the colinearity rules. HOTTIP RNA knockdown abates both the H3K4me3 level and the expression of the 5′ HoxA genes in a distance dependent fashion but has no effects on the higher-order spatial compaction of the locus. At the same time, expression of the HoxD genes is not affected. Together, this indicates that HOTTIP lincRNA acts in cis, close to the site where it is synthesized. HOTTIP lincRNA directly interacts with the WDR5, a component TrxG protein complex responsible for methylation of H3K4 and thus recruits the latter to the neighboring genes. Depletion of HOTTIP lincRNA with short-hairpin RNAs in chicken embryonic limb buds leads to developmental defects similar to those caused by mutations in the 5′ HoxA genes,Citation13 suggesting that HOTTIP lincRNA is important for the regulation of activity of the Hox genes during embryogenesis. Due to its expression pattern, the HOTTIP lincRNA appears not be involved in the triggering of the transcription of the Hox genes, but rather participates in the maintenance of the established status, perhaps by shifting the balance between H3K27me3 and H3K4me3.
Hox in Space: Outlook
The bipartite spatial organization of chromatin provides an attractive model for the strictly ordered regulation of the Hox clusters. Assuming that the activity of a gene is influenced by its spatial neighbors, it is now easy to imagine that the expression status of an individual Hox gene can be altered by shifting it from one compartment to the other. The colinear organization of the Hox clusters may facilitate such transitions in an ordered fashion.
However, we note that the causal relationships among gene activity, chromatin states, and spatial folding still need to be resolved. Most importantly, is the bipartite 3D organization essential for the regulation of the Hox genes, or is it merely a passive consequence of the intrinsic propensity of different chromatin types to self-aggregate? And are the two main chromatin states indeed essential for establishment of the two compartments, or is it the other way around? Like most chickens and eggs in biology, the causal relationships may well turn out to be circular.
Acknowledgments
We thank Jacqueline Deschamps, Natalia Soshnikova, Elena Kozhevnikova and members of our laboratory for critical reading of the manuscript. This work was supported by the Netherlands Consortium for Systems Biology.
References
- Spitz F, Gonzalez F, Duboule D. A global control region defines a chromosomal regulatory landscape containing the HoxD cluster. Cell 2003; 113:405 - 17; http://dx.doi.org/10.1016/S0092-8674(03)00310-6; PMID: 12732147
- Fraser P, Bickmore W. Nuclear organization of the genome and the potential for gene regulation. Nature 2007; 447:413 - 7; http://dx.doi.org/10.1038/nature05916; PMID: 17522674
- Dekker J, Rippe K, Dekker M, Kleckner N. Capturing chromosome conformation. Science 2002; 295:1306 - 11; http://dx.doi.org/10.1126/science.1067799; PMID: 11847345
- Dostie J, Richmond TA, Arnaout RA, Selzer RR, Lee WL, Honan TA, et al. Chromosome Conformation Capture Carbon Copy (5C): a massively parallel solution for mapping interactions between genomic elements. Genome Res 2006; 16:1299 - 309; http://dx.doi.org/10.1101/gr.5571506; PMID: 16954542
- Simonis M, Klous P, Splinter E, Moshkin Y, Willemsen R, de Wit E, et al. Nuclear organization of active and inactive chromatin domains uncovered by chromosome conformation capture-on-chip (4C). Nat Genet 2006; 38:1348 - 54; http://dx.doi.org/10.1038/ng1896; PMID: 17033623
- Würtele H, Chartrand P. Genome-wide scanning of HoxB1-associated loci in mouse ES cells using an open-ended Chromosome Conformation Capture methodology. Chromosome Res 2006; 14:477 - 95; http://dx.doi.org/10.1007/s10577-006-1075-0; PMID: 16823611
- Tiwari VK, Cope L, McGarvey KM, Ohm JE, Baylin SB. A novel 6C assay uncovers Polycomb-mediated higher order chromatin conformations. Genome Res 2008; 18:1171 - 9; http://dx.doi.org/10.1101/gr.073452.107; PMID: 18502945
- Lieberman-Aiden E, van Berkum NL, Williams L, Imakaev M, Ragoczy T, Telling A, et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science 2009; 326:289 - 93; http://dx.doi.org/10.1126/science.1181369; PMID: 19815776
- van Steensel B, Dekker J. Genomics tools for unraveling chromosome architecture. Nat Biotechnol 2010; 28:1089 - 95; http://dx.doi.org/10.1038/nbt.1680; PMID: 20944601
- Sutherland H, Bickmore WA. Transcription factories: gene expression in unions?. Nat Rev Genet 2009; 10:457 - 66; http://dx.doi.org/10.1038/nrg2592; PMID: 19506577
- Guelen L, Pagie L, Brasset E, Meuleman W, Faza MB, Talhout W, et al. Domain organization of human chromosomes revealed by mapping of nuclear lamina interactions. Nature 2008; 453:948 - 51; http://dx.doi.org/10.1038/nature06947; PMID: 18463634
- Németh A, Conesa A, Santoyo-Lopez J, Medina I, Montaner D, Péterfia B, et al. Initial genomics of the human nucleolus. PLoS Genet 2010; 6:e1000889; http://dx.doi.org/10.1371/journal.pgen.1000889; PMID: 20361057
- Wang KC, Yang YW, Liu B, Sanyal A, Corces-Zimmerman R, Chen Y, et al. A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature 2011; 472:120 - 4; http://dx.doi.org/10.1038/nature09819; PMID: 21423168
- Noordermeer D, Leleu M, Splinter E, Rougemont J, De Laat W, Duboule D. The dynamic architecture of Hox gene clusters. Science 2011; 334:222 - 5; http://dx.doi.org/10.1126/science.1207194; PMID: 21998387
- Montavon T, Soshnikova N, Mascrez B, Joye E, Thevenet L, Splinter E, et al. A regulatory archipelago controls hox genes transcription in digits. Cell 2011; 147:1132 - 45; http://dx.doi.org/10.1016/j.cell.2011.10.023; PMID: 22118467
- Favier B, Dollé P. Developmental functions of mammalian Hox genes. Mol Hum Reprod 1997; 3:115 - 31; http://dx.doi.org/10.1093/molehr/3.2.115; PMID: 9239717
- Richardson L, Venkataraman S, Stevenson P, Yang Y, Burton N, Rao J, et al. EMAGE mouse embryo spatial gene expression database: 2010 update. Nucleic Acids Res 2010; 38:Database issue D703 - 9; http://dx.doi.org/10.1093/nar/gkp763; PMID: 19767607
- Kmita M, Duboule D. Organizing axes in time and space; 25 years of colinear tinkering. Science 2003; 301:331 - 3; http://dx.doi.org/10.1126/science.1085753; PMID: 12869751
- Rinn JL, Bondre C, Gladstone HB, Brown PO, Chang HY. Anatomic demarcation by positional variation in fibroblast gene expression programs. PLoS Genet 2006; 2:e119; http://dx.doi.org/10.1371/journal.pgen.0020119; PMID: 16895450
- Schuettengruber B, Chourrout D, Vervoort M, Leblanc B, Cavalli G. Genome regulation by polycomb and trithorax proteins. Cell 2007; 128:735 - 45; http://dx.doi.org/10.1016/j.cell.2007.02.009; PMID: 17320510
- Soshnikova N, Duboule D. Epigenetic temporal control of mouse Hox genes in vivo. Science 2009; 324:1320 - 3; http://dx.doi.org/10.1126/science.1171468; PMID: 19498168
- Chambeyron S, Bickmore WA. Chromatin decondensation and nuclear reorganization of the HoxB locus upon induction of transcription. Genes Dev 2004; 18:1119 - 30; http://dx.doi.org/10.1101/gad.292104; PMID: 15155579
- Chambeyron S, Da Silva NR, Lawson KA, Bickmore WA. Nuclear re-organisation of the Hoxb complex during mouse embryonic development. Development 2005; 132:2215 - 23; http://dx.doi.org/10.1242/dev.01813; PMID: 15829525
- Fraser J, Rousseau M, Shenker S, Ferraiuolo MA, Hayashizaki Y, Blanchette M, et al. Chromatin conformation signatures of cellular differentiation. Genome Biol 2009; 10:R37; http://dx.doi.org/10.1186/gb-2009-10-4-r37; PMID: 19374771
- Ferraiuolo MA, Rousseau M, Miyamoto C, Shenker S, Wang XQ, Nadler M, et al. The three-dimensional architecture of Hox cluster silencing. Nucleic Acids Res 2010; 38:7472 - 84; http://dx.doi.org/10.1093/nar/gkq644; PMID: 20660483
- Grau DJ, Chapman BA, Garlick JD, Borowsky M, Francis NJ, Kingston RE. Compaction of chromatin by diverse Polycomb group proteins requires localized regions of high charge. Genes Dev 2011; 25:2210 - 21; http://dx.doi.org/10.1101/gad.17288211; PMID: 22012622
- Eskeland R, Leeb M, Grimes GR, Kress C, Boyle S, Sproul D, et al. Ring1B compacts chromatin structure and represses gene expression independent of histone ubiquitination. Mol Cell 2010; 38:452 - 64; http://dx.doi.org/10.1016/j.molcel.2010.02.032; PMID: 20471950
- Misteli T. Higher-order genome organization in human disease. Cold Spring Harb Perspect Biol 2010; 2:a000794; http://dx.doi.org/10.1101/cshperspect.a000794; PMID: 20591991
- Tschopp P, Duboule D. A genetic approach to the transcriptional regulation of Hox gene clusters. Annu Rev Genet 2011; 45:145 - 66; http://dx.doi.org/10.1146/annurev-genet-102209-163429; PMID: 22060042
- Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X, Brugmann SA, et al. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell 2007; 129:1311 - 23; http://dx.doi.org/10.1016/j.cell.2007.05.022; PMID: 17604720
- Schorderet P, Duboule D. Structural and functional differences in the long non-coding RNA hotair in mouse and human. PLoS Genet 2011; 7:e1002071; http://dx.doi.org/10.1371/journal.pgen.1002071; PMID: 21637793