Abstract
Pre-replication complexes (pre-RCs) are assembled onto DNA during late mitosis and G1 to license replication origins for use in S phase. In order to prevent re-replication of DNA, licensing must be completely shutdown prior to entry into S phase. While mechanisms preventing re-replication during S phase and mitosis have been elucidated, the means by which cells first prevent licensing during late G1 phase are poorly understood. We have employed a hybrid mammalian / Xenopus egg extract replication system to dissect activities that inhibit replication licensing at different stages of the cell cycle in Chinese Hamster Ovary (CHO) cells. We find that soluble extracts from mitotic cells inhibit licensing through a combination of geminin and Cdk activities, while extracts from S-phase cells inhibit licensing predominantly through geminin alone. Surprisingly however, geminin did not accumulate until after cells enter S phase. Unlike extracts from cells in early G1 phase, extracts from late G1 phase and early S phase cells contained an inhibitor of licensing that could not be accounted for by either geminin or Cdk. Moreover, inhibiting cyclin and geminin protein synthesis or inhibiting Cdk activity early in G1 phase did not prevent the appearance of inhibitory activity. These results suggest that a soluble inhibitor of replication licensing appears prior to entry into S phase that is distinct from either geminin or Cdk activity. Our hybrid system should permit the identification of this and other novel cell cycle regulatory activities.
Introduction
DNA replication must be regulated to duplicate the genome faithfully and exactly once per cell cycle. This is achieved in eukaryotes by two mutually exclusive alternating periods of the cell cycle.Citation1–Citation4 Pre-replication complex (pre-RC) assembly culminates in the loading of the Mcm2–7 helicase during telophase to license origins for initiation at a time when initiation of replication is prevented. Replication initiates during S phase under conditions in which licensing is prevented. In mammalian cells, licensing is inhibited during S phase and G2 predominantly by preventing the activity of the essential licensing protein Cdt1 through a combination of proteolysis and sequestering of Cdt1 by binding to the licensing inhibitor geminin. In mitosis, licensing is also inhibited by high Cdk activity that prevents pre-RC proteins from binding to DNA. Licensing takes place during telophase,Citation5–Citation9 shortly after the degradation of both cyclins and geminin, which are maintained at low levels during G1 phase by the APC/Cdh1 ubiquitin ligase complex.
It is critical that pre-RC assembly be shut down completely prior to entry into S phase in order to prevent any re-replication. There is currently a gap in our knowledge as to exactly when licensing ceases during G1 phase in preparation for S phase. One key event could be the loss of APC/Cdh1 activity during G1 phase, which allows geminin levels to rise.Citation10 However, APC/Cdh1 inactivation also results in the elevation of cyclins, and if this were the sole means of regulation, there would be a risk that rising Cdk activity could drive initiation before geminin had reached a sufficiently high level. The origin decision point (ODP), the time at which a subset of pre-RCs are selected for initiation potential, occurs prior to the R-point and it has been hypothesized that cessation of pre-RC assembly and eviction of pre-RCs by other chromosomal activities such as transcription could account for the ODP.Citation11 In fact, selection of origins at the ODP is sensitive to Cdk2 inhibitors,Citation12 although Cdk2 activity has not been detected until the R-point.Citation12,Citation13
Here, we have investigated the utility of Xenopus egg extracts, in which replication activities can be readily manipulated, to evaluate licensing and pre-RC assembly during the mammalian cell cycle. This cross-species complementation approach allows the dissection of mammalian cell cycle activities using Xenopus egg extracts. We find that extracts from mitotic cells inhibit Mcm2–7 protein loading through a combination of geminin and Cdk activity as expected. However, extracts from S-phase cells inhibit licensing exclusively through geminin, with no detectable contribution from Cdk activity. Finally, we present evidence for the presence of a soluble replication inhibitor that accumulates during the pre-restriction point stage of G1 phase to inhibit pre-RC assembly, but is distinct from either Cdk or geminin.
Results
Geminin accumulation and Cdt1 degradation occur after the onset of S phase.
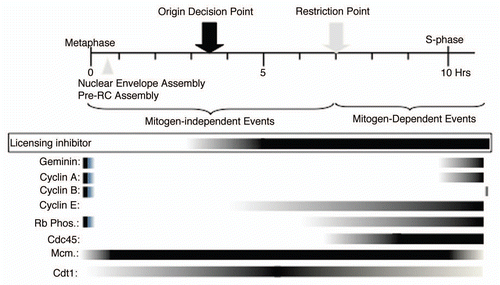
In several earlier publications, we have carefully staged the timing of G1-phase events using CHO cells that can be synchronized to near homogeneity by mitotic shake-off. Degradation of cyclins A and B and geminin occur within 10 minutes after release from a brief (4 hour) nocodazole block,Citation7 and the accumulation of Mcm2–7 on chromatin begins 20–30 minutes later, coincident with the ability of chromatin from such cells to replicate in an Mcm-depleted or geminin-supplemented Xenopus egg extract.Citation6,Citation7,Citation14 Mcm2–7 proteins continue to accumulate on chromatin throughout G1 phase, with more than half the total Mcm2–7 in the cell associating with chromatin by the onset of S phase.Citation14,Citation15 The Origin Decision Point (ODP) occurs between 3 and 4 hours after mitosis,Citation16 whereas the R-point—defined by either mitogen-independent entry of cells into S phase (point of no return) or phosphorylation of the Retinoblastoma tumor suppressor protein (Rb)—occurs between 6 and 8 hours after mitosis.Citation17 Cells staining positive for PCNA focal staining or BrdU incorporation, indicating entry into S phase, can be first seen around the time of initial Rb phosphorylation. Cells continue to enter S phase over many hours, with approximately 50% of cells in S phase by 12 hours, while cyclin A accumulation occurs between 10–12 hours after mitosis.Citation7,Citation15,Citation17
To place geminin and Cdt1 protein synthesis into this timeline, we performed immuno-blotting of either whole cell extracts or chromatin-containing fractions prepared from CHO cells synchronized at various time points after mitosis (). Chromatin-bound PCNA (as well as BrdU incorporation; example shown in ) served as an indicator of entry into S phase and, consistent with prior results summarized above, appeared by 6 hours post-mitosis. Also consistent with prior work, cyclin A appeared (weakly) 4–6 hours after the first cells entered S and cyclin B appeared much later into S phase. Cyclin E (which had not been previously analyzed) appeared just prior to the R-point and diminished late into S phase, consistent with the initial phosphorylation of Rb and entry into S phase being mediated by cyclinE-Cdk2 complexes.Citation17 Surprisingly, the appearance of geminin and the degradation of Cdt1 on chromatin also did not occur until 4–6 hours after the first cells entered S both, coincident with the appearance of cyclin A. We also noticed a transient and reproducible increase in Cdt1 levels in both whole cell extract and chromatin fraction 5–6 hours after mitosis. Overall, the timing of both the appearance of geminin and the degradation of Cdt1 cannot account for the prevention of pre-RC assembly prior to entry into S phase. Cyclin E however, remained a viable candidate for such an activity.
Early G1 phase is uniquely permissive for replication licensing.
We wished to devise an assay to detect inhibitors of Mcm loading within extracts from mammalian cells synchronized at different times during G1 phase. To do so, we took advantage of a previously described assay for replication licensing.Citation18,Citation19 This two-step assay () recapitulates the two distinct phases of the cell cycle in which: (1) licensing but not initiation can occur and; (2) initiation but not licensing can occur. By first carrying out the licensing reaction using Xenopus sperm chromatin as a substrate and a soluble Xenopus egg extract (“High Speed Supernatant”; HSS) to elicit the licensing reaction (Step 1), pre-RC proteins assemble onto the sperm chromatin but a nuclear envelope is unable to assemble, which is necessary for the accumulation of Cdk activity required for the initiation step. The efficiency of licensing can then be evaluated by adding to the licensing reaction a sufficient quantity of complete Xenopus extract supplemented with high concentration of non-degradable geminin (Step 2). Complete extracts have the ability to form a functional nucleus around licensed chromatin that leads to accumulation and concentration of Cdk activity required for initiation. At the same time, the supplemented non-degradable geminin prevents the complete extracts from licensing origins using Xenopus proteins. Hence, DNA synthesis observed in Step 2 is a read out of the successful licensing (i.e., Mcm loading) of chromatin in Step 1. Under standard Xenopus replication conditions, a large excess of Mcm proteins assemble onto chromatin and more than 90% of Mcm proteins must be depleted to observe measurable effects on DNA synthesis.Citation20,Citation21 Thus, to increase the sensitivity of the DNA synthesis readout to inhibitors of Mcm loading, we titrated the amount of template Xenopus sperm DNA introduced into the initial licensing reaction in Step 1 to identify a concentration of sperm DNA at which Mcm loading would be limiting for subsequent DNA synthesis in Step 2 (). A linear relationship between DNA input and DNA synthesis was found between 20–80 ng sperm DNA per microliter of licensing reaction. Hence, 40 ng per microliter was used in Step 1 for all subsequent experiments. Direct measurement of Mcm loading (which requires considerably more extract and was not practical as a routine assay), confirmed that DNA synthesis was an accurate readout of Mcm loading under these experimental conditions ().
To evaluate licensing activity during the mammalian cell cycle, soluble cellular extracts were made from Chinese Hamster Ovary (CHO) cells synchronized at various times during the cell cycle and tested for their ability to completely substitute for Xenopus proteins in the licensing step of the reaction. Extracts were prepared from cells in metaphase (M), 2 hr after nocodazole release (early G1; pre-ODP), 5–7 hr after nocodazole release (late G1; post-ODP), and after release from mitosis for 16 hours in the presence of the replication inhibitor aphidicolin, followed by a 1 hour release from aphidicolin (S). We found that none of these extracts had licensing activity on their own (). This was expected, since ORC and Cdc6 proteins, essential for licensing, are found exclusively in the chromatin fraction in CHO cells throughout the cell cycle under these extraction conditions and so are not present in our soluble CHO cell extracts.Citation7,Citation22 To confirm that the lack of licensing activity was due to selective absence of ORC and Cdc6 proteins in these soluble extracts, we separately immunodepleted ORC, Cdc6, Mcm and Cdt1 proteins from Xenopus egg extracts and evaluated the ability of soluble CHO extracts to rescue loss of these individual pre-RC components (). As expected, none of the mammalian extracts could compliment the loss of ORC or Cdc6. However, extracts from early G1-phase cells, but not late G1-phase, M-phase or S-phase cells, could rescue licensing activity in Cdt1- or Mcm-depleted extracts. The inability of extracts other than from early G1 cells to complement Cdt1 or Mcm2–7 activity was not due simply to the selective absence of Cdt1 or Mcm proteins, as these proteins were present in similar amounts in soluble extracts from all of these time points ().
We next wanted to distinguish whether the inability of extracts from late G1-phase, M-phase or S-phase cells to complement depleted extracts was due to the absence of licensing activity or to the presence of an inhibitor of licensing. To that end, we performed mixing experiments in which various ratios of hamster to Xenopus egg extract were used during the licensing step of the reaction. If components were simply missing from some of the mammalian cell extracts, mixing should not inhibit licensing more than the addition of buffer. Results ( and B) revealed that hamster extracts from all but early G1-phase contain an inhibitory activity. Moreover, extracts prepared at different times during the cell cycle had different levels of inhibitory activity manifest by the ratio of Xenopus to hamster cell extract required to detect the inhibition of licensing. S-phase extracts showed strong inhibition even at low concentrations, while M-phase and late G1-phase extracts required a higher ratio of hamster to Xenopus extract. Importantly, early G1 phase showed little or no inhibitory activity at any ratio.
Extracts from mitotic cells inhibit the loading of Mcm2–7 proteins through a combination of geminin and Cdk activity.
We first considered whether the inhibitory activity of the different extracts was due to known pathways that inhibit licensing during the cell cycle. To this end, we sought to separately neutralize the two known inhibitors of licensing: geminin and Cdk activity. Geminin can be neutralized using a peptide derivative of Cdt1 (Cdt1193–447), which retains the geminin interacting domain of Cdt1 but lacks the ability to participate in the licensing reaction.Citation23 Cdt1193–447 can titrate geminin from extracts and prevent it from inhibiting licensing. Cdk activity can be neutralized using the inhibitor 6-dimethylaminopurine (6-DMAP). Although the specificity of 6-DMAP for Cdk vs. other protein kinases is not as defined as other Cdk inhibitors such as roscovitine,Citation24 we have shown that unlike other Cdk inhibitors, 6-DMAP only weakly inhibits the initiation of DNA replication in Xenopus egg extractsCitation25–Citation27 allowing us to evaluate DNA synthesis in the read out reaction. We began by examining extracts from mitotic cells, previously shown to have high levels of both geminin and cyclins.Citation7 Cdt1193–447 and/or 6-DMAP were added to extracts made from CHO cells synchronized in mitosis prior to their addition to the Xenopus egg extract licensing reaction. As shown in , both Cdt1193–447 and 6-DMAP were able to partially neutralize the licensing inhibition activity in mitotic extracts while the effects of Cdt1193–447 and 6-DMAP were additive for relieving inhibition.
To confirm that the inhibition of licensing activity reflects the efficiency with which Mcm2–7 proteins were loaded onto chromatin,Citation18 chromatin was isolated from extracts following the licensing reaction and analyzed by immuno-blotting with anti-Mcm antibodies. Using this assay we could make use of roscovitine as a more specific inhibitor of Cdk activity because we did not need to maintain the initiation-competence of the extracts. As shown in , the loading of both Mcm3 and Mcm7 was strongly inhibited when mitotic extracts were mixed into the licensing reaction. However, the addition of either Cdt1193–447 or roscovitine partially rescued Mcm loading and the addition of both rescued Mcm loading to the levels seen without the addition of any mitotic extract. We conclude that the inhibition of licensing by mitotic extracts is due to the prevention of Mcm2–7 loading by the combined activities of Cdk and geminin.
Extracts from S-phase cells inhibit the loading of Mcm2–7 exclusively through geminin.
Next, we examined the inhibitory activity in extracts from S-phase cells, which also contain high amounts of geminin and cyclin A, but not cyclin B ( and reviewed in refs. Citation7 and Citation17). While Cdt1193–447 neutralized a large fraction of the licensing inhibitory activity in S-phase extracts, 6-DMAP had no effect, even in combination with Cdt1193–447 (). Similarly, when Mcm loading was examined directly, neutralizing geminin with Cdt1193–447 completely restored Mcm loading, whereas roscovitine treatment did not result in any detectable increase in Mcm loading (). Similar results were obtained whether CHO cells were synchronized at 14 hours after release from mitotic selection (60% cells in S-phase) or were arrested at the G1/S boundary with an aphidicolin block and released for one hour (not shown). Hence, the inhibition of licensing by S-phase extracts is primarily due to the inhibition of Mcm2–7 loading by geminin. This is consistent with previous results showing that geminin, but not Cdks, plays a major role in inhibiting licensing during S phase and G2 Citation8,Citation28–Citation31 and extends these findings to suggest that geminin is solely responsible for the inhibition of licensing during this phase of the cell cycle.
A pre-restriction point inhibitor of licensing.
To further investigate the licensing inhibitory activity found in extracts from late G1-phase cells, we first analyzed extracts from cells synchronized at hourly intervals after mitosis. Results (), demonstrated that the licensing inhibitory activity first appeared between 3 and 4 hours and increased until 6 hours. Late G1-phase extracts also detectably inhibited the loading of Mcm proteins onto chromatin (). These cells are upstream of phosphorylation of the retinoblastoma protein and passage through the R-point, which takes place 6–8 hours after mitosisCitation17 but partially overlaps the ODP, which takes place between 3–5 hours.Citation16,Citation32 Cell cycle events during G1-phase are highly reproducible in this cell line under the synchronization conditions used here, and the hallmarks shown in are all consistent with prior work. Moreover, monitoring the entry of cells into S-phase of the same cells used in these experiments by scoring the percentage of cells incorporating 5-bromo-2-deoxyuridine (BrdU) in a brief pulse label at each time point verified that cells were moving through G1-phase at a rate consistent with all of our prior cell cycle studies in this cell line ().
Several observations confirm that the late G1-phase inhibitory activity is not due to partial contamination of G1-phase cells with cells that had entered S phase prematurely. First, there is no significant increase in inhibitory activity during the 7–9 hour period () when the percentage of S-phase cells increases from 7 to 27% (). Second, mixing S-phase extracts with early G1-phase extracts (prepared 2 hours after mitosis) revealed that inhibition equivalent to that seen with late G1-phase extracts would require at least 30% S-phase extract contamination (). Third, the data shown in and D demonstrate that S-phase licensing inhibition is almost totally accounted for by geminin. However, we could not detect any geminin present in extracts prepared as late as 9 hours after mitosis (), when more than 25% of cells were already in S-phase (). In fact, geminin was not detectable in whole cell extracts until 10–12 hours after mitosis (). Moreover, Cdt1193-447 was unable to neutralize the inhibitory activity of late G1-phase extracts (, E and F), suggesting that geminin does not play a major role in preventing licensing during late G1. Interestingly, 6-DMAP addition was also unable to neutralize the late G1-phase licensing inhibition (, E and F), as it did with M-phase extracts ( and B), suggesting that this activity is not due to Cdk activity. Together, these results indicate that during the pre-restriction point period of G1-phase, at or near the ODP, an activity distinct from geminin or Cdk arises that can inhibit replication licensing.
What could be the nature of this pre-restriction point licensing inhibitor? One candidate would be Cdt1 or Cdc6 degradation. However, Cdc6 is equally associated with chromatin throughout the cell cycle in CHO cellsCitation7 and there is actually a transient increase in both total and chromatin-bound Cdt1 levels during the initial appearance of the inhibitory activity (). Moreover, we did not detect any degradation of Xenopus Cdt1 when mammalian extracts were added to the licensing reaction (Sasaki T, unpublished). One previously described but poorly characterized activity that could potentially influence licensing is Mcm acetylation.Citation33,Citation34 However, we found that the addition of a pan-acetylase inhibitor (acetonyl Co-A) or a pan-HDAC inhibitor (TSA or butyrate) did not affect Mcm loading onto chromatin in the licensing reaction (Sasaki T, unpublished). This result would also appear to rule out the HBO1 acetylase that is required for replication licensing.Citation35,Citation36
Late G1 phase licensing inhibition does not require Cdk activity or protein synthesis.
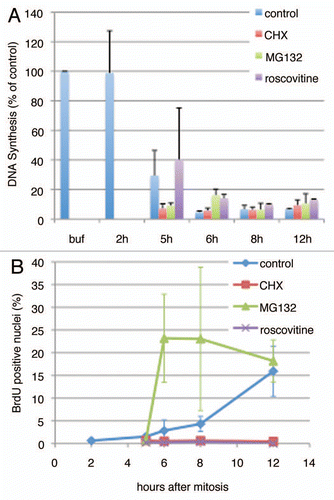
In order to investigate the relationship between this novel activity and origin selection at the ODP, we performed the licensing inhibition assay using the hamster extracts prepared from cells allowed to progress from early to late G1 in the presence of the Cdk inhibitor roscovitine, the proteolysis inhibitor MG132 or the protein synthesis inhibitor cycloheximide (). Roscovitine and MG132 both inhibit passage of cells through the ODP, while cycloheximide does not.Citation12 All three of these drugs have strong effects on the entry of cells into S phase (). Both roscovitine and cycloheximide prevented entry into S phase, consistent with their ability to prevent passage through the restriction point.Citation12,Citation17,Citation37 Interestingly, MG132 caused a precocious entry of cells into S phase and rapid appearance of a licensing inhibitory activity. Both of these observations can be accounted for by the stabilization and accumulation of both cyclins and geminin by MG132, both of which are normally degraded by APCCdh1. This suggests that MG132 inhibition of the ODP is due to precocious initiation of replication prior to origin choice, but is not informative to the appearance of the late G1 phase inhibitory activity. We do not understand why cycloheximide appeared to enhance inhibitory activity; it is possible that the continuous synthesis of a protein during early G1 prevents the appearance of the inhibitory activity. Nonetheless, inhibition of licensing cannot result from Cdk or geminin activity since their synthesis was prevented. Most importantly however, neither roscovitine nor cycloheximide significantly affected the appearance of a late G1 licensing inhibition activity, indicating that late G1 phase licensing inhibition is independent of the ODP. The fact that the licensing inhibitory activity appears in the absence of protein (and thus cyclin and geminin) synthesis and in the presence of a Cdk inhibitor provides supporting evidence that the activity is not due to geminin or Cdk activity. These results also confirm that the activity is upstream of the R-point. In fact, extracts from cells arrested in quiescence also display licensing inhibition that is independent of Cdk and geminin (Sasaki T, unpublished).
Discussion
We describe a system to identify novel replication regulatory activities in extracts from mammalian cells. We have employed this system to dissect activities that affect replication licensing at different stages of the mammalian cell cycle. We find that extracts from mitotic cells inhibit Mcm2–7 protein loading through a combination of geminin and Cdk activity as expected. However, extracts from S-phase cells inhibit licensing exclusively through geminin, with no detectable contribution from Cdk activity. As expected from previous biochemical analysis, inhibition of licensing is accounted for by the prevention of Mcm2–7 protein loading onto DNA.Citation18,Citation19 Finally, we demonstrate the presence of an unexpected and previously uncharacterized soluble replication inhibitor that accumulates during the pre-restriction point stage of G1-phase. This inhibitor is distinct from either geminin or Cdk activity. Our novel hybrid assay will permit the testing of candidates for this activity and possibly its eventual identification.
While the combined inhibitory activities of Cdk and geminin in mitotic extracts were expected (albeit we have not distinguished Cdk1 from Cdk2 activities in our experiments), it was quite surprising to find that the S-phase pre-RC assembly activity, which was the strongest inhibitory activity during the cell cycle, was almost entirely accounted for by geminin with no detectable contribution from Cdk activity. Cdk activity is required for S phase progression and cyclins are abundantly present at the times our extracts were prepared. In fact, we have previously detected robust Cdk activity in soluble cellular extracts from S-phase cells.Citation13 Despite this, our experiments demonstrate that licensing inhibition is largely independent of Cdk activity. Inhibition of Cdk either in the in vitro licensing reaction or in cultured cells prior to preparation of extracts had no effect on late G1/Early S phase inhibition of licensing. Hence, our results indicate that S phase Cdk activity is neither necessary nor sufficient to inhibit Mcm loading in mammalian cells.
Our results raise the question: what prevents pre-RCs from re-forming on newly synthesized chromatin during early S-phase prior to the appearance of geminin or Cdk activity? The obvious known candidates summarized in have so far not provided productive leads. Our experiments suggest that the activity does not involve degradation of licensing factors Cdt1 or Cdc6. Our experimental results are also not consistent with appearance of an Mcm acetylase activity nor inhibition of the HBO1 acetylase, activities that have both been implicated in the licensing reaction. However, the assay described here should permit the purification of more defined fractions of soluble extracts that will facilitate identification of the unknown inhibitor.
Materials and Methods
Xenopus extracts and de-membranated Xenopus sperm chromatin.
LSS (low-speed supernatant) and HSS (high-speed supernatant) from Xenopus eggs and de-membranated Xenopus sperm chromatin were prepared as previously described in reference Citation38. Immuno-depleted Xenopus extracts were prepared as described in reference Citation38 and Citation39.
Hamster soluble extracts for licensing, Mcm loading, protein stability assay.
Hamster CHOC400 cells were cultured and synchronized as previously described in reference Citation40. Cells were harvested and washed twice in ice-cold LFB 1/50 (50 mM KCl, 40 mM Hepes-KOH pH 8.0, 20 mM potassium phosphate pH 8.0, 2 mM MgCl2, 1 mM EGTA, 10% sucrose, 2 mM DTT, 1 ug/ml each of pepstatin, aprotinin, leupeptin, 0.5 mM PMSF), resuspended in ice-cold LFB 1/50 at 107 cells/60 µl, then disrupted by three cycles of freezing and thawing. The lysate was spun at full speed in a microcentrifuge for one minute at 4°C and the supernatant was collected. This procedure resulted in extract protein concentrations >20 mg/ml. Extracts were adjusted to 20 mg/ml protein with LFB 1/50 and frozen in liquid nitrogen until use. Synchronization of cells was confirmed by either metaphase spread analysis (for M phase cells) or BrdU incorporation (for G1- and S-phase cells), as described in reference Citation40.
Antibodies and western blotting.
Whole cell extracts and chromatin fractions from CHO cells were prepared as previously described in reference Citation7. Isolation of Xenopus sperm chromatin from the licensing reaction was performed as described in reference Citation41. In order to detect Xenopus proteins, rabbit anti-Xenopus Mcm3,Citation42 rabbit anti-Xenopus Mcm7,Citation42 rabbit anti-Xenopus Cdt1Citation43 were used. In order to detect hamster proteins, anti-human geminin (Santa Cruz, sc-13015), anti-human Cdt1 (guinea pig #47, gift from Jean Cook), anti-human cyclin A (Santa Cruz, sc-596), anti-human cyclin B1 (Santa Cruz, sc-245), anti-human cyclin E (Santa Cruz, sc-481), anti-human PCNA (Oncogene NA03-200UG), anti-human Mcm7 (Santa Cruz, sc-9966) were used. Anti-human histone H3 (Abcam, ab1791) was used for both hamster and Xenopus histone H3 for loading control. For the immuno-depletion of Xenopus egg extract, anti-Xenopus ORC1,Citation44 anti-anti-Xenopus Mcm3,Citation42 anti-Xenopus Cdt1,Citation43 anti-Xenopus Cdc6 Citation45 were used. Coomassie staining of histones () was performed as described in reference Citation46.
Licensing/licensing inhibition assay.
The principle of licensing/licensing inhibition assay is described in the text and , and has been previously described in reference Citation18 and Citation19. For mixing experiments, the ratio of Xenopus HSS to hamster soluble extract in licensing reaction was optimized for each synchronization experiment as follows. For M and S phase inhibitory activity, the minimum amount of hamster extract to give maximal licensing inhibition was chosen, which was 1:1 for M phase and 3:1 for S phase. For G1 phase inhibitory activity, we chose the ratio to detect the maximum licensing inhibition for late G1 relative to early G1 extracts, which was 1:3. Crude Xenopus ORC, Cdc6, Mcm and Cdt1 fractions were prepared by selective PEG precipitation described in reference Citation18 and Citation43. Cdt1 193–447 is described in reference Citation43. After the licensing reaction, the appropriate volume of Xenopus LSS was added in Step 2 to achieve a final sperm concentration of 13.3 ng DNA/µl LSS.
Disclosure of Potential Conflict of Interest
No potential conflicts of interest were disclosed.
Figures and Tables
Figure 1 Geminin Protein Accumulates After Entry Into S Phase. CHO cells were synchronized in mitosis by shake-off and released into the cell cycle for the indicated times. Either whole cell extracts (WCE ) or a chromatin-containing cell fraction (chromatin) was subjected to western blotting with the indicated antibodies. Cell cycle hallmarks discussed in the text are indicated above the figure.
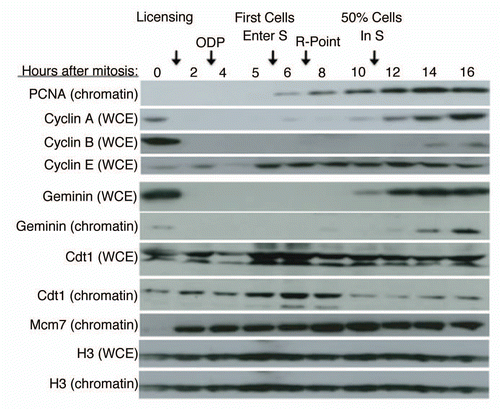
Figure 2 A heterologous system for the identification of licensing activities. (A) Description of licensing assay protocol (see text and methods for details). (B) Various concentrations of sperm chromatin were added to soluble high-speed Xenopus extract in Step 1, followed by the addition of sufficient geminin-supplemented complete (low-speed) extract to maintain a constant sperm template concentration in Step 2 of 13.3 ng/µl. The relative fraction of input DNA synthesized was plotted as a function of original template concentration in Step 1. This experiment identifies the Step 1 template concentration at which licensing components (e.g., Mcm2–7) become limiting for DNA synthesis. 40 ng/µl was used in all subsequent experiments. (C) Immediately following Step 1 from (B), sperm chromatin was isolated from aliquots of the reaction by centrifugation through a sucrose cushion. Chromatin bound proteins from equivalent amounts of sperm DNA from each reaction were subjected to immuno-blotting to detect the amount of chromatin-loaded Mcm proteins. (D) In Step 1, soluble high-speed Xenopus egg extract was replaced with either Buffer (Buf) or soluble extracts from CHO cells at different times during the cell cycle (M = mitosis or cells at shake-off; Early G1 = 2 hours after mitosis; Late G1 = 7 hours after mitosis; S = S phase; cells released from mitosis into aphidicolin for 16 hours and released from the aphidicolin block for one hour). CHO extracts cannot license Xenopus sperm chromatin on their own, regardless of the time during the cell cycle at which they are prepared. (E) Soluble high-speed Xenopus egg extracts were depleted of ORC1, Cdc6, Mcm3 or Cdt1 and Step 1 of the licensing reaction was performed with mixtures of depleted extract and either Buffer (Buf) or CHO cell extracts (Xenopus: Mammalian extract ratio 1:3) prepared at the indicated times during the cell cycle as in (D). As positive control reactions, depleted Xenopus extracts were supplemented with partially purified fractions of Xenopus egg extract (Crude Xen.: PEG-M for Mcm3 depleted extracts and PEG-B for all others; reference Citation18) and all reactions were quantified relative to this positive control. (F) Soluble hamster extracts used in (E) were subjected to immuno-blotting to examine the level of hamster Cdt1 and Mcm proteins.
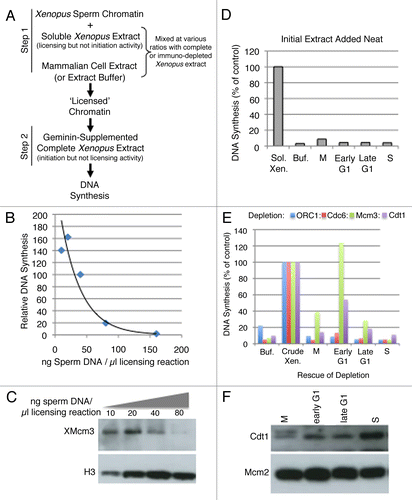
Figure 3 Inhibition of licensing by hamster extracts. (A) Various ratios of high-speed Xenopus extract: mammalian extract or buffer (Buf.) were mixed with 40 ng/µl sperm chromatin during Step 1 of the licensing reaction, followed by the addition of sufficient geminin-supplemented complete (low-speed) extract to maintain a constant sperm template concentration in Step 2 of 13.3. ng/µl, as in . Licensing inhibition is shown as a percentage of the DNA synthesis seen relative to the maximum value when the assay was performed with high-speed Xenopus extract:buffer = 3:1. (B) The experiment shown in (A) was repeated with 3 independent batches of Xenopus and CHO extracts. For M and S phase reactions, the “Optimal Ratio” of Xenopus:Mammalian extract was determined as the minimum amount of hamster extract to give maximal inhibition, which was 1:1 for M phase and 3:1 for S phase. For G1 phase inhibitory activity, the “Optimal Ratio” was the ratio to detect the maximum licensing inhibition for late G1 relative to early G1 extracts, which was 1:3.
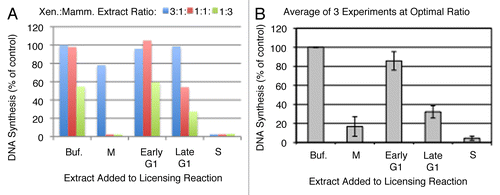
Figure 4 Nature of licensing inhibitor in M phase and S phase hamster extracts. (A and C) Step 1 of the licensing assay was performed with highspeed Xenopus extract mixed with buffer alone (grey) or with M phase (A; ratio of 1:1) or S phase (C; ratio of 3:1) CHO extract (white). Prior to mixture, buffer or CHO extracts were supplemented with the Cdk inhibitor 6-DMAP (3 mM), a geminin-neutralizing fragment of Cdt1 (amino acids 193–447; Con7, 5 ng/µl), or both inhibitors simultaneously. DNA synthesis in Step 2 is expressed as a percentage of that obtained with buffer alone. Shown are the means of three experiments with independent extract batches and error bars show standard deviation. (B and D) Xenopus sperm chromatin was incubated with high-speed Xenopus extract mixed with buffer (+Buffer) or hamster M-phase (B) or S-phase (D) extract supplemented with inhibitors as in (A and C) except that Roscovitine (rosco, 40 µM) was substituted for 6-DMAP as a more potent and specific inhibitor of Cdk activity. Following the 25 min Step 1 incubation period, sperm chromatin was isolated and subjected to immuno-blotting to evaluate Mcm protein loading.
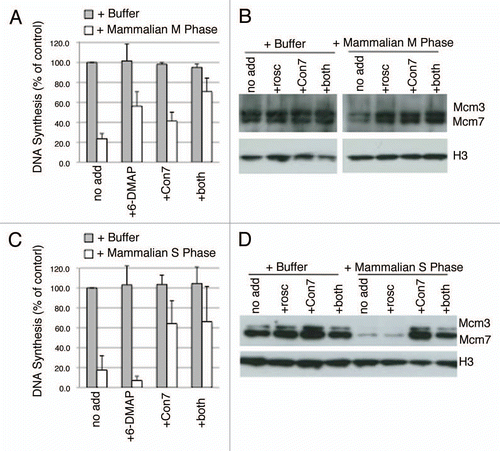
Figure 5 Nature of licensing inhibitor in late G1 hamster extracts. (A) Similar to and C, Step 1 of the licensing assay was performed with high-speed Xenopus extract mixed with buffer alone (Buf) or with extracts from CHO cells synchronized at the indicated times after mitosis at a 1:3 ratio. Prior to mixture, buffer or CHO extracts were supplemented with 6-DMAP (red), or Cdt1193–447 (green). DNA synthesis in Step 2 is expressed as a percentage of that obtained with buffer alone. Shown are the results of two independent assays and the standard deviation. Below the graph is an immuno-blot showing the Mcm2 and geminin levels in the soluble hamster extracts from each time point, as well as M and S phase from the same synchronized cells. (B) Xenopus sperm chromatin was incubated with high-speed Xenopus extract mixed with buffer (+Buffer) or extracts from CHO cells synchronized at the indicated times after mitosis. Following the 25 min Step 1 incubation period, sperm chromatin was isolated and subjected to immuno-blotting to evaluate the relative amounts of Mcm loading after incubation with extracts from each G1 phase time point (A). In this experiment, histones were detected by coomassie staining. (C) Prior to collection, aliquots of each of the cell preparations from (A) were pulse labeled with BrdU and the percentage in S phase cells was evaluated. (D) Step 1 of the licensing reaction was performed with high-speed Xenopus extract mixed at a 1:1 ratio with buffer (+Buffer) or extracts from CHO cells synchronized either 2 hours after mitosis (Early G1 Phase) or after release from aphidicolin as in – (S Phase), as well as combinations of these two CHO cell extracts mixed at the indicated ratios. This demonstrates that 10–33% contamination of S-phase cells would be needed to detect licensing inhibition. (E) Similar to and C, Step 1 of the licensing assay was performed with high-speed Xenopus extract mixed at a ratio of 1:3 with buffer alone (grey) or with extracts from CHO cells synchronized 7 hours after mitosis (Late G1 Phase; white). Prior to mixture, buffer or CHO extracts were supplemented with the Cdk inhibitor 6-DMAP , Cdt1193–447 (Con7), or both inhibitors simultaneously. DNA synthesis in Step 2 is expressed as a percentage of that obtained with buffer alone. Shown are the means of three experiments with independent extract batches and error bars show standard deviation. (F) Similar to and D, Xenopus sperm chromatin was incubated with high-speed Xenopus extract mixed at a ratio of 1:3 with buffer alone (+Buffer) or extracts from CHO cells synchronized 7 hours after mitosis (+Mammalian G1 Phase) supplemented with inhibitors as in (E) except that Roscovitine (rosco) was substituted for 6-DMAP as a more potent and specific inhibitor of Cdk activity, as in and D. Following the 25 min Step 1 incubation period, sperm chromatin was isolated and subjected to western blotting to evaluate Mcm protein loading. Neither Roscovitine nor Cdt1193–447 (Con7) could recover Mcm loading to levels seen with Buffer alone.
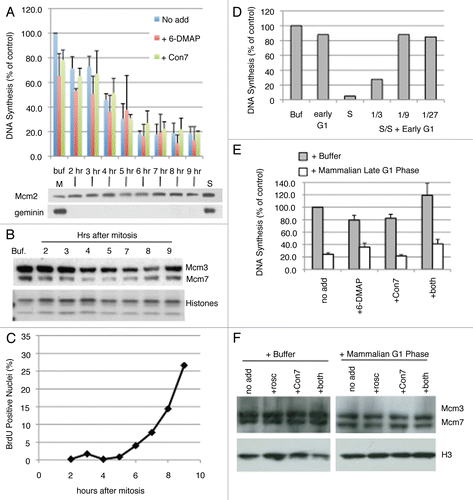
Figure 6 Drug effect on late G1 licensing inhibition. (A) Step 1 of the licensing assay was carried out mixing high-speed Xenopus extract at a 1:3 ratio with either Buffer (Buf) or soluble extracts from CHO cells synchronized at the indicated times after mitosis. Cells were treated with the indicated inhibitors (cycloheximide, CHX 50 µg/ml; MG132, 10 µM; roscovitine, 40 µM) starting from 2 hours after mitosis. Shown are the average of two independent assays and the standard deviation. (B) Progression into S phase for each of the CHO cell cultures used to prepare extracts in (A) was monitored by BrdU pulse labeling as in .
Acknowledgments
We regretfully and respectfully acknowledge the passing of A. Li during the course of these studies. We thank J. Cook for anti human Cdt1 antibody and P. Cole for acetonyl-CoA. This work was funded by National Institute for General Medical Sciences grant GM083337 to D.M.G., CRUK grants C303/A7399 and C303/A5434 to J.J.B., A.L. and P.J.G. and BBSRC grant BB/H013024/1 to P.J.G.
References
- Masai H, Matsumoto S, You Z, Yoshizawa-Sugata N, Oda M. Eukaryotic chromosome DNA replication: where, when and how?. Annu Rev Biochem 2010; 79:89 - 130
- Xouri G, Lygerou Z, Nishitani H, Pachnis V, Nurse P, Taraviras S. Cdt1 and geminin are downregulated upon cell cycle exit and are overexpressed in cancer-derived cell lines. Eur J Biochem 2004; 271:3368 - 3378
- Blow JJ, Dutta A. Preventing re-replication of chromosomal DNA. Nat Rev Mol Cell Biol 2005; 6:476 - 486
- Arias EE, Walter JC. Strength in numbers: preventing rereplication via multiple mechanisms in eukaryotic cells. Genes Dev 2007; 21:497 - 518
- Mendez J, Stillman B. Chromatin association of human origin recognition complex, cdc6 and minichromosome maintenance proteins during the cell cycle: assembly of prereplication complexes in late mitosis. Mol Cell Biol 2000; 20:8602 - 8612
- Dimitrova DS, Prokhorova TA, Blow JJ, Todorov IT, Gilbert DM. Mammalian nuclei become licensed for DNA replication during late telophase. J Cell Sci 2002; 115:51 - 59
- Okuno Y, McNairn AJ, den Elzen N, Pines J, Gilbert DM. Stability, chromatin association and functional activity of mammalian pre-replication complex proteins during the cell cycle. EMBO J 2001; 20:4263 - 4277
- Li A, Blow JJ. Cdt1 downregulation by proteolysis and geminin inhibition prevents DNA re-replication in Xenopus. EMBO J 2005; 24:395 - 404
- Namdar M, Kearsey SE. Analysis of Mcm2–7 chromatin binding during anaphase and in the transition to quiescence in fission yeast. Exp Cell Res 2006; 312:3360 - 3369
- Qiao X, Zhang L, Gamper AM, Fujita T, Wan Y. APC/C-Cdh1: from cell cycle to cellular differentiation and genomic integrity. Cell Cycle 2010; 9:3904 - 3912
- Sasaki T, Ramanathan S, Okuno Y, Kumagai C, Shaikh SS, Gilbert DM. The Chinese hamster dihydrofolate reductase replication origin decision point follows activation of transcription and suppresses initiation of replication within transcription units. Mol Cell Biol 2006; 26:1051 - 1062
- Keezer SM, Gilbert DM. Sensitivity of the origin decision point to specific inhibitors of cellular signaling and metabolism. Exp Cell Res 2002; 273:54 - 64
- Keezer SM, Gilbert DM. Evidence for a pre-restriction point Cdk3 activity. J Cell Biochem 2002; 85:545 - 552
- Dimitrova DS, Todorov IT, Melendy T, Gilbert DM. Mcm2, but not RPA, is a component of the mammalian early G1-phase pre-replication complex. J Cell Biol 1999; 146:709 - 722
- Kuipers MA, Stasevich TJ, Sasaki T, Wilson KA, Hazelwood KL, McNally JG, et al. Highly stable loading of Mcm proteins onto chromatin in living cells requires replication to unload. J Cell Biol 2011; 192:29 - 41
- Wu JR, Gilbert DM. A distinct G1 step required to specify the chinese hamster DHFR replication origin. Science 1996; 271:1270 - 1272
- Wu JR, Gilbert DM. The replication origin decision point is a mitogen independent, 2-aminopurine sensitve, G1-phase step that precedes restriction point control. Mol Cell Biol 1997; 17:4312 - 4321
- Chong JPJ, Mahbubani HM, Khoo CY, Blow JJ. Purification of an MCM-containing complex as a component of the DNA replication licensing system. Nature 1995; 375:418 - 424
- Gillespie PJ, Li A, Blow JJ. Reconstitution of licensed replication origins on Xenopus sperm nuclei using purified proteins. BMC Biochem 2001; 2:15
- Mahbubani H, Chong J, Chevalier S, Thommes P, Blow J. Cell cycle regulation of the replication licensing system: involvement of a Cdk-dependent inhibitor. J Cell Biol 1997; 136:125 - 135
- Walter J, Newport JW. Regulation of replicon size in Xenopus egg extracts. Science 1997; 275:993 - 995
- McNairn AJ, Gilbert DM. Overexpression of ORC subunits and increased ORC-chromatin association in transformed mammalian cells. J Cell Biochem 2005;
- Ferenbach A, Li A, Brito-Martins M, Blow JJ. Functional domains of the Xenopus replication licensing factor Cdt1. Nucleic Acids Res 2005; 33:316 - 324
- Meijer L, Borgne A, Mulner O, Chong JP, Blow JJ, Inagaki N, et al. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur J Biochem 1997; 243:527 - 536
- Blow JJ. Preventing re-replication of DNA in a single cell cycle: evidence for a replication licensing factor. J Cell Biol 1993; 122:993 - 1002
- Luciani MG, Oehlmann M, Blow JJ. Characterization of a novel ATR-dependent, Chk1-independent, intra-S-phase checkpoint that suppresses initiation of replication in Xenopus. J Cell Sci 2004; 117:6019 - 6030
- Li A, Blow JJ. Negative regulation of geminin by CDK-dependent ubiquitination controls replication licensing. Cell Cycle 2004; 3:443 - 445
- Quinn LM, Herr A, McGarry TJ, Richardson H. The Drosophila Geminin homolog: roles for Geminin in limiting DNA replication, in anaphase and in neurogenesis. Genes Dev 2001; 15:2741 - 2754
- Mihaylov IS, Kondo T, Jones L, Ryzhikov S, Tanaka J, Zheng J, et al. Control of DNA replication and chromosome ploidy by geminin and cyclin A. Mol Cell Biol 2002; 22:1868 - 18680
- Melixetian M, Ballabeni A, Masiero L, Gasparini P, Zamponi R, Bartek J, et al. Loss of Geminin induces rereplication in the presence of functional p53. J Cell Biol 2004; 165:473 - 482
- Zhu W, Chen Y, Dutta A. Rereplication by depletion of geminin is seen regardless of p53 status and activates a G2/M checkpoint. Mol Cell Biol 2004; 24:7140 - 7150
- Dimitrova D, Gilbert D. Regulation of mammalian replication origin usage in Xenopus egg extracts. J Cell Sci 1998; 111:2989 - 2998
- Takei Y, Assenberg M, Tsujimoto G, Laskey R. The MCM3 acetylase MCM3AP inhibits initiation, but not elongation, of DNA replication via interaction with MCM3. J Biol Chem 2002; 277:43121 - 43125
- Takei Y, Swietlik M, Tanoue A, Tsujimoto G, Kouzarides T, Laskey R. MCM3AP, a novel acetyltransferase that acetylates replication protein MCM3. EMBO Rep 2001; 2:119 - 123
- Miotto B, Struhl K. HBO1 histone acetylase activity is essential for DNA replication licensing and inhibited by Geminin. Mol Cell 2010; 37:57 - 66
- Miotto B, Struhl K. HBO1 histone acetylase is a coactivator of the replication licensing factor Cdt1. Genes Dev 2008; 22:2633 - 2638
- Wu JR, Keezer S, Gilbert D. Transformation abrogates an early G1-phase arrest point required for specification of the Chinese hamster DHFR replication origin. EMBO J 1998; 17:1810 - 1818
- Chong J, Thommes P, Rowles A, Mahbubani H, Blow J. Characterisation of the Xenopus replication licensing system. Methods Enzymol 1997; 283:549 - 564
- Oehlmann M, Score AJ, Blow JJ. The role of Cdc6 in ensuring complete genome licensing and S phase checkpoint activation. J Cell Biol 2004; 165:181 - 190
- Wu JW, Yu G, Gilbert DM. Origin-Specific Initiation of Mammalian Nuclear DNA Replication in a Xenopus Cell-Free System. METHODS, A Companion to Methods In Enzymology 1997; 13:313 - 324
- Rowles A, Tada S, Blow JJ. Changes in association of the Xenopus origin recognition complex with chromatin on licensing of replication origins. J Cell Sci 1999; 112:2011 - 2018
- Prokhorova TA, Blow JJ. Sequential MCM/P1 subcomplex assembly is required to form a heterohexamer with replication licensing activity. J Biol Chem 2000; 275:2491 - 2498
- Tada S, Li A, Maiorano D, Mechali M, Blow JJ. Repression of origin assembly in metaphase depends on inhibition of RLF-B/Cdt1 by geminin. Nat Cell Biol 2001; 3:107 - 113
- Rowles A, Chong J, Brown L, Howell M, Evan G, Blow J. Interaction between the origin recognition complex and the replication licensing system in Xenopus. Cell 1996; 87:287 - 296
- Tada S, Chong JPJ, Mahbubani HM, Blow JJ. The RLF-B component of the replication licensing system is distinct from Cdc6 and functions after Cdc6 binds to chromatin. Curr Biol 1999; 9:211 - 214
- Jares P, Luciani MG, Blow JJ. A Xenopus Dbf4 homolog is required for Cdc7 chromatin binding and DNA replication. BMC Mol Biol 2004; 5:5