Abstract
Cohesin and cohesin regulatory proteins function in an essential pathway enabling proper cohesion and segregation of sister chromatids. Additionally, these proteins are involved in double-strand break (DSB) repair and transcriptional regulation. Mutations in Establishment of cohesion 1 homolog 2 (Esco2), an evolutionary conserved cohesin acetyltransferase, are the cause of Roberts syndrome (RBS), a human congenital disorder. To explore the mechanism by which the deficiency in Esco2 affects cohesin’s functions, we generated a mouse harboring a conditional Esco2 allele. To our surprise and in marked contrast to RBS, mouse Esco2 turns out to be a cell viability factor, the absence of which results in severe chromosome segregation defects and apoptosis. We found that the acetylation of the cohesin subunit Smc3 is significantly reduced in Esco2-deficient cells resulting in a marked reduction of Sororin recruitment to several, but not all cohesin bound loci. Here, we provide evidence that Esco2 is also required for DSB repair, which is consistent with previous studies in RBS cells.
Accurate segregation of mitotic chromosomes in eukaryotic cells hinges on cohesion between sister chromatids. Cohesion is established in S phase at which time sister chromatids are generated by DNA replication and remain linked until their separation in anaphase. Sister chromatid cohesion, is mediated by the ring-like protein complex, cohesin, composed of Smc1, Smc3, Rad21 and SA1 or SA2 subunits.Citation1,Citation2 The establishment of cohesion is thought to be associated with the acetylation of the Smc3 subunitCitation3-Citation6 during S phase. In higher organisms Smc3 acetylation has been linked to the recruitment of SororinCitation7 which increases of stability of cohesin’s association with chromatin.Citation8 In yeast, Smc3 acetylation is essential for viability and depends on an evolutionary conserved acetyltransferase, Eco1.Citation3,Citation4 Mammalian genomes encode two Eco1 orthologs, Esco1 and Esco2, characterized by a highly divergent N-terminus followed by a C2H2 zinc finger and an acetyltransferase domain.Citation9 Both enzymes have been shown to acetylate Smc3.Citation7 A deficiency in ESCO2 in humans causes Roberts syndrome (RBS),Citation10,Citation11 characterized by craniofacial and limb dysmorphologies. RBS patients can survive to adulthood, even though many of them harbor ESCO2 loss-of-function mutations.Citation10 Typically, prometaphase chromosomes from RBS cells show loss of cohesion at and near the centromere, but the sister chromatids retain their parallel alignment and show a “railroad track” appearance.Citation12
In view of the RBS findings, it was surprising that our recent work in the mouse showed that Esco2 deficiency leads to a loss-of-viability in early preimplantation embryos, which failed to develop beyond the second cell division. We had established a mouse genetic model containing a floxed Esco2 allele, which allowed us the deletion of Esco2 in a tissue-specific manner. Deletion of Esco2 in cortical progenitors led to cortical agenesis since Esco2-deficient neural progenitors underwent cell death.
Reminiscent of RBS cells, mouse embryonic fibroblasts deficient for Esco2 (MEFsEsco2Δ/Δ) are characterized by parallel alignment of sister chromatids in prometaphase chromosomes, but in contrast to RBS cells, MEFsEsco2Δ/Δ show severe chromosome segregation defects resulting in highly abnormal nuclear morphology.Citation13 It is likely that the observed chromosome segregation defects in MEFsEsco2Δ/Δ primarily result from their inability to properly establish cohesion between the sister chromatids. Support for this view comes from our finding that in the absence of Esco2, the acetylation of Smc3 and the recruitment of Sororin, which is essential for sister chromatid cohesion, was > 50% diminished from mid S phase until prometaphase, with the most striking reduction in Sororin binding at pericentric heterochromatin (PCH). Previous studies, using knock-down techniques in HeLa cells, suggested a partial redundancy of ESCO1 and ESCO2, since in such cells detectable changes in acetylation of SMC3 required depletion of both acetyltransferases.Citation7 This discrepancy with our studies underlines the usefulness of mouse genetic models in the analysis of cohesion mechanisms. Taken together, our results thus suggest that Esco2 has an essential function in the establishment of sister chromatid cohesion, which cannot be substituted by Esco1.
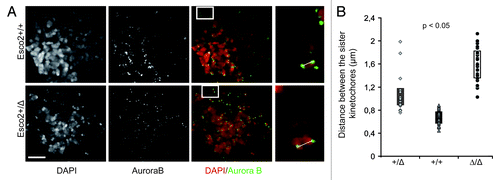
We noticed that the deletion of single copy of the Esco2 gene as in MEFsEsco2+/Δ leads to a small, but measurable increase in inter-kinetochore distance in prometaphase chromosomes (0.696 ± 0.132 ∝m in MEFsEsco2+/+ vs. 0.988 ± 0.212 ∝m in MEFsEsco2+/Δ and 1.66 ± 0.273 ∝m in MEFsEsco2Δ/Δ, and ). Despite the increase in distance between the sister kinetochores, heterozygosity in Esco2 does neither significantly impact chromosome segregation nor affect embryonic development. Heterozygous animals are born at expected frequency and do not show any developmental malformations.Citation13
Figure 1. Deletion in single Esco2 allele leads to measurable increase in the interkinetochore distance. (A) Prometaphase chromosomes from wild type (Esco2+/+) and heterozygous (Esco2Δ/+) MEFs were labeled with anti-Aurora B antibody to visualize sister kinetochores. Boxed areas depict a single chromosome in high magnification. The distance between the sisters kinetochores (white line), the position of which was determined by the peak Aurora B fluorescence, was measured in deconvoluted samples using Leica Advance Fluorescence software. The mean values from individual cells were plotted in graph (B). Scale bars-5 μm and 1 μm, n > 200, p value was obtained using Mann-Whitney U-test.
Additionally, our recent study provides first insights toward the molecular mechanism leading to the railroad track appearance of the chromosomes in MEFsEsco2Δ/Δ. This cytological phenotype might involve diminished phosphorylation of histone H2A-threonine 120 at the kinetochore in MEFsEsco2Δ/Δ. This would prevent proper targeting of the cohesin protector Shugoshin (Sgo1) to the centromere.Citation14 Indeed, we found that prometaphase chromosomes in MEFsEsco2Δ/Δ show a reduction in Sgo1 immunofluorescence at the centromere. Interestingly, Sgo1 became localized to the chromosome arms. This change in Sgo1 localization may de-protect centromeres from the cohesin-removing prophase pathway and instead protect of cohesin at the chromosome arms. This view is consistent with the finding that the distribution of cohesin along the prometaphase chromosomes in MEFsEsco2Δ/Δ is rather uniform, explaining also the parallel alignment of sister chromatids.Citation13
It is possible that the defect in Sgo1 recruitment to the kinetochores is an indirect consequence of deficiency in PCH cohesion in MEFsEsco2Δ/Δ. We speculate that PCH cohesin lacking the stabilizing factor Sororin dissociates in prophase. This could affect the establishment of chromatin marks at the kinetochore, which are necessary for proper targeting of chromosomal passenger complex proteins and shugoshin to the centromere.Citation15-Citation17 The existence of a direct link between chromatin bound cohesin and histone modification was demonstrated for Haspin-mediated phosphorylation of Histone 3 at threonine 3.Citation17 This however needs to be analyzed in more detail in Esco2-deficient cells. Taken together, we envisage two mechanisms, which are mutually not exclusive and which may both contribute to the cytogenetic phenotype of cells lacking Esco2. One is the failure to properly establish PCH cohesion in S phase. The other may be based on defects in cohesin protection at the kinetochore.
Although Smc3 acetylation and Sororin binding are markedly reduced in MEFsEsco2Δ/Δ, both are still detectable by immunological methods. The residual Smc3 acetylation may be performed by Esco1. We envisage two hypotheses explaining the non-redundant function of Esco2. The “dosage hypothesis” presumes that the amount of enzymatic activity provided by Esco1 might be insufficient to adequately acetylate Smc3 and hence, cohesion at critical chromosomal regions is impaired. Support for this hypothesis comes from our finding that PCH and a locus at the arm of chromosome 11, both of which are characteristically bound by high amounts of cohesin, show marked reduction in Sororin recruitment in Esco2-deficient cells.Citation13 One would predict that overexpression of Esco1 should rescue or at least ameliorate the Esco2-deficiency.
The “regional specificity hypothesis” is based on the finding that in somatic vertebrate cells there are at least two distinct cohesin populations which differ from each other by the presence of either the SA1 or the SA2 subunit.Citation18,Citation19 While SA1-cohesin is specifically required for telomere cohesion,Citation20,Citation21 depletion of SA2Citation20—similar to the depletion of Esco2Citation13—abolishes the cohesion at the centromeres. SA2-cohesin and Esco2 thus show overlapping “regional” specificity, which could result from higher affinity of Esco2 to a SA2-cohesin substrate. To substantiate the regional specificity hypothesis, it will be necessary to examine the levels of Smc3 acetylation in MEFsEsco2Δ/Δ in SA1- and SA2-cohesins separately.
Additionally, future work should directly determine sites and levels of Smc3 acetylation at cohesin-bound-loci on a genome-wide scale. At the present time, we used Sororin ChIP as surrogate readout for Smc3 acetylation. There is an agreement between our acetylation data and findings based on Sororin immunofluorescence and ChIP. While Smc3 acetylation was described to occur from yeast to mammals,Citation3,Citation4,Citation22 Sororin or a functional ortholog has not been identified in the yeast genome yet.Citation7 This raises the question of whether Smc3 acetylation in mammalian cells by Esco1 and Esco2 serves purposes additional to Sororin recruitment. For example, it will be interesting to compare MEFs from Sororin-deficient mice with Esco1/Esco2 double mutant mice with regard to cohesion and gene regulation which has been shown to be regulated in part by cohesin’s association with CTCFCitation23 or Mediator.Citation24 One reason for addressing the gene regulation issue is our observation that Smc3 acetylation was already detected in the G1 phase of the cell cycle, at which time there is no SororinCitation8 but gene expression takes place.
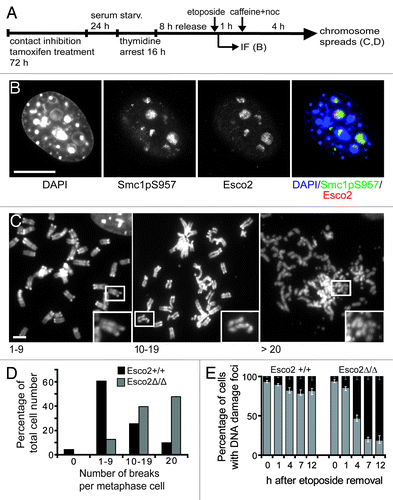
Similar to RBS cells,Citation25 yeast strains with Roberts syndrome mimicking mutation in Eco1’s acetyltransferase domain (eco1-W216G) show only mild cohesion defects, but have a severe deficiency in DNA damage repair.Citation26 During the double strand break (DSB) repair, new cohesion has to be formed in order to ensure close proximity between the broken chromatid and the repair template.Citation27 Moreover, even a limited number of DSBs will induce genome wide, postreplicative reinforcement of sister chromatid cohesion in Eco1-dependent manner.Citation28,Citation29 Consistent with a role of Eco1 in DSB repair and DSB-induced cohesion enforcement, we found that upon the induction of DSBs by etoposide or bleomycine treatment in late S/G2 () the bulk of Esco2 protein localizes to the sites of the DNA lesion (). Our data further shows that MEFsEsco2Δ/Δ are characterized by a high frequency of chromosome fragmentation () and the persistence of histone H2AX gamma positive foci (), indicating that these cells are deficient in the repair of damaged DNA. Taken together these data suggest that Esco2 is required for DNA repair and this may be so for two reasons. In the absence of Esco2 cohesion defects could prevent homologous recombination and DSB repair. Second, Esco2 could be required for acetylation of Smc3 or another substrateCitation30 directly at the site of DSB, where we observed high amount of Esco2 protein accumulation.
Figure 2. Esco2 localizes to the sites of DNA double strand breaks and is required for the efficient DNA DSB repair. (A) Experimental scheme. Esco2 was depleted in contact inhibited/serum starved MEFs by tamoxifen treatment. Cells were then arrested at the G1/S boundary using thymidine block. Eight h after release into S phase, double strand breaks were induced using etoposide (5 μM) or bleomycine (20 μgml-1) treatment. DNA damage checkpoint was blocked by caffeine (2 mM). (B) Efficiency of DBS induction was monitored by immunofluorescence (IF) using an antibody raised against Smc1 phosphorylated at S957 (Rockland, clone 5D11G5, dilution 1:50). In wild type MEFs, Esco2 localized to the sites of DBS marked with Smc1pS957 IF. Scale bar 8 μm. (C) Prometaphase chromosomes isolated from wild type and Esco2-deficient MEFs were divided into four groups according to the number of breaks: chromosomes without the breaks, chromosome spreads containing between 1–9 breaks, chromosome spreads containing between 10–19 breaks and chromosome spreads containing more than 20 breaks. Examples of prometaphase chromosomes with 1–9, 10–19 and more than 20 breaks are depicted. Boxed areas are magnified in insets. Scale bar 3 μm. (D) Esco2 is required for efficient DSB repair. The frequencies of prometaphase chromosomes with 0, 1–9, 10–19 and more than 20 breaks were plotted into the bar graph (n > 500). (E) Esco2-deficient MEFs are characterized by the persistence of DNA damage foci. DSB were induced 8 h after thymidine block release and the presence of DNA damage foci was monitored on the basis H2A.XpS139 IF (Abcam, ab22551, dilution 1:200) 1, 4, 7 and 12 h after the etoposide withdrawal. The frequency of H2A.XpS139 positive (black) and H2A.XpS139 negative (gray) cells was plotted (n > 500, per group and time point).
Collectively, our recent study reveals that the cohesin acetyltransferases Esco1 and Esco2 have non-redundant functions. The paradox why human mutations in this gene do not cause lethality remains to be resolved. Alternative transcripts or suppressor mutations could contribute to generally milder cytological phenotype and survival of RBS patients and their presence in the patient samples needs to be examined. Future work should be also directed at genome-wide changes in Smc3 acetylation and/or Sororin binding in cells lacking Esco1 and/or Esco2. Mouse lines enabling conditional inactivation of Esco1 and Esco2 genes will be indispensable tools to address this issue. They would also provide an entry way in the question of whether Esco1/2 proteins contribute to the non-canonical function of cohesin.
Acknowledgments
The research leading to these results has received funding from the Max Planck Society, Boehringer Ingelheim, the Austrian Science Fund (FWF; special research program SFB F34 “Chromosome Dynamics”), the Vienna Science and Technology Fund (WWTF NS09-13), the Austrian Ministry for Science and Research (GEN-AU program ‘Epigenetic control’) and the European Community’s Seventh Framework Programme (FP7/2007-2013) under grant agreement n° 241548 (MitoSys).
References
- Onn I, Heidinger-Pauli JM, Guacci V, Unal E, Koshland DE. Sister chromatid cohesion: a simple concept with a complex reality. Annu Rev Cell Dev Biol 2008; 24:105 - 29; http://dx.doi.org/10.1146/annurev.cellbio.24.110707.175350; PMID: 18616427
- Nasmyth K, Haering CH. Cohesin: its roles and mechanisms. Annu Rev Genet 2009; 43:525 - 58; http://dx.doi.org/10.1146/annurev-genet-102108-134233; PMID: 19886810
- Rolef Ben-Shahar T, Heeger S, Lehane C, East P, Flynn H, Skehel M, et al. Eco1-dependent cohesin acetylation during establishment of sister chromatid cohesion. Science 2008; 321:563 - 6; http://dx.doi.org/10.1126/science.1157774; PMID: 18653893
- Unal E, Heidinger-Pauli JM, Kim W, Guacci V, Onn I, Gygi SP, et al. A molecular determinant for the establishment of sister chromatid cohesion. Science 2008; 321:566 - 9; http://dx.doi.org/10.1126/science.1157880; PMID: 18653894
- Rowland BD, Roig MB, Nishino T, Kurze A, Uluocak P, Mishra A, et al. Building sister chromatid cohesion: smc3 acetylation counteracts an antiestablishment activity. Mol Cell 2009; 33:763 - 74; http://dx.doi.org/10.1016/j.molcel.2009.02.028; PMID: 19328069
- Sutani T, Kawaguchi T, Kanno R, Itoh T, Shirahige K. Budding yeast Wpl1(Rad61)-Pds5 complex counteracts sister chromatid cohesion-establishing reaction. Curr Biol 2009; 19:492 - 7; http://dx.doi.org/10.1016/j.cub.2009.01.062; PMID: 19268589
- Nishiyama T, Ladurner R, Schmitz J, Kreidl E, Schleiffer A, Bhaskara V, et al. Sororin mediates sister chromatid cohesion by antagonizing Wapl. Cell 2010; 143:737 - 49; http://dx.doi.org/10.1016/j.cell.2010.10.031; PMID: 21111234
- Schmitz J, Watrin E, Lénárt P, Mechtler K, Peters JM. Sororin is required for stable binding of cohesin to chromatin and for sister chromatid cohesion in interphase. Curr Biol 2007; 17:630 - 6; http://dx.doi.org/10.1016/j.cub.2007.02.029; PMID: 17349791
- Hou FJ, Zou H. Two human orthologues of Eco1/Ctf7 acetyltransferases are both required for proper sister-chromatid cohesion. Mol Biol Cell 2005; 16:3908 - 18; http://dx.doi.org/10.1091/mbc.E04-12-1063; PMID: 15958495
- Vega H, Waisfisz Q, Gordillo M, Sakai N, Yanagihara I, Yamada M, et al. Roberts syndrome is caused by mutations in ESCO2, a human homolog of yeast ECO1 that is essential for the establishment of sister chromatid cohesion. Nat Genet 2005; 37:468 - 70; http://dx.doi.org/10.1038/ng1548; PMID: 15821733
- Schüle B, Oviedo A, Johnston K, Pai S, Francke U. Inactivating mutations in ESCO2 cause SC phocomelia and Roberts syndrome: no phenotype-genotype correlation. Am J Hum Genet 2005; 77:1117 - 28; http://dx.doi.org/10.1086/498695; PMID: 16380922
- Maserati E, Pasquali F, Zuffardi O, Buttitta P, Cuoco C, Defant G, et al. Roberts syndrome: phenotypic variation, cytogenetic definition and heterozygote detection. Ann Genet 1991; 34:239 - 46; PMID: 1809233
- Whelan G, Kreidl E, Wutz G, Egner A, Peters JM, Eichele G. Cohesin acetyltransferase Esco2 is a cell viability factor and is required for cohesion in pericentric heterochromatin. EMBO J 2012; 31:71 - 82; http://dx.doi.org/10.1038/emboj.2011.381; PMID: 22101327
- Kawashima SA, Yamagishi Y, Honda T, Ishiguro K, Watanabe Y. Phosphorylation of H2A by Bub1 prevents chromosomal instability through localizing shugoshin. Science 2010; 327:172 - 7; http://dx.doi.org/10.1126/science.1180189; PMID: 19965387
- Wang F, Dai J, Daum JR, Niedzialkowska E, Banerjee B, Stukenberg PT, et al. Histone H3 Thr-3 phosphorylation by Haspin positions Aurora B at centromeres in mitosis. Science 2010; 330:231 - 5; http://dx.doi.org/10.1126/science.1189435; PMID: 20705812
- Kelly AE, Ghenoiu C, Xue JZ, Zierhut C, Kimura H, Funabiki H. Survivin reads phosphorylated histone H3 threonine 3 to activate the mitotic kinase Aurora B. Science 2010; 330:235 - 9; http://dx.doi.org/10.1126/science.1189505; PMID: 20705815
- Yamagishi Y, Honda T, Tanno Y, Watanabe Y. Two histone marks establish the inner centromere and chromosome bi-orientation. Science 2010; 330:239 - 43; http://dx.doi.org/10.1126/science.1194498; PMID: 20929775
- Losada A, Yokochi T, Kobayashi R, Hirano T. Identification and characterization of SA/Scc3p subunits in the Xenopus and human cohesin complexes. J Cell Biol 2000; 150:405 - 16; http://dx.doi.org/10.1083/jcb.150.3.405; PMID: 10931856
- Sumara I, Vorlaufer E, Gieffers C, Peters BH, Peters JM. Characterization of vertebrate cohesin complexes and their regulation in prophase. J Cell Biol 2000; 151:749 - 62; http://dx.doi.org/10.1083/jcb.151.4.749; PMID: 11076961
- Canudas S, Smith S. Differential regulation of telomere and centromere cohesion by the Scc3 homologues SA1 and SA2, respectively, in human cells. J Cell Biol 2009; 187:165 - 73; http://dx.doi.org/10.1083/jcb.200903096; PMID: 19822671
- Remeseiro S, Cuadrado A, Carretero M, Martínez P, Drosopoulos WC, Cañamero M, et al. Cohesin-SA1 deficiency drives aneuploidy and tumourigenesis in mice due to impaired replication of telomeres. EMBO J 2012; 31:2076 - 89; http://dx.doi.org/10.1038/emboj.2012.11; PMID: 22415365
- Zhang JL, Shi XM, Li YH, Kim BJ, Jia JL, Huang ZW, et al. Acetylation of Smc3 by Eco1 is required for S phase sister chromatid cohesion in both human and yeast. Mol Cell 2008; 31:143 - 51; http://dx.doi.org/10.1016/j.molcel.2008.06.006; PMID: 18614053
- Wendt KS, Yoshida K, Itoh T, Bando M, Koch B, Schirghuber E, et al. Cohesin mediates transcriptional insulation by CCCTC-binding factor. Nature 2008; 451:796 - 801; http://dx.doi.org/10.1038/nature06634; PMID: 18235444
- Kagey MH, Newman JJ, Bilodeau S, Zhan Y, Orlando DA, van Berkum NL, et al. Mediator and cohesin connect gene expression and chromatin architecture. Nature 2010; 467:430 - 5; http://dx.doi.org/10.1038/nature09380; PMID: 20720539
- van der Lelij P, Godthelp BC, van Zon W, van Gosliga D, Oostra AB, Steltenpool J, et al. The cellular phenotype of Roberts syndrome fibroblasts as revealed by ectopic expression of ESCO2. PLoS One 2009; 4:e6936; http://dx.doi.org/10.1371/journal.pone.0006936; PMID: 19738907
- Lu S, Goering M, Gard S, Xiong B, McNairn AJ, Jaspersen SL, et al. Eco1 is important for DNA damage repair in S. cerevisiae.. Cell Cycle 2010; 9:3315 - 27; http://dx.doi.org/10.4161/cc.9.16.12673; PMID: 20703090
- Sjögren C, Nasmyth K. Sister chromatid cohesion is required for postreplicative double-strand break repair in Saccharomyces cerevisiae.. Curr Biol 2001; 11:991 - 5; http://dx.doi.org/10.1016/S0960-9822(01)00271-8; PMID: 11448778
- Ström L, Karlsson C, Lindroos HB, Wedahl S, Katou Y, Shirahige K, et al. Postreplicative formation of cohesion is required for repair and induced by a single DNA break. Science 2007; 317:242 - 5; http://dx.doi.org/10.1126/science.1140649; PMID: 17626884
- Unal E, Heidinger-Pauli JM, Koshland D. DNA double-strand breaks trigger genome-wide sister-chromatid cohesion through Eco1 (Ctf7). Science 2007; 317:245 - 8; http://dx.doi.org/10.1126/science.1140637; PMID: 17626885
- Heidinger-Pauli JM, Unal E, Koshland D. Distinct targets of the Eco1 acetyltransferase modulate cohesion in S phase and in response to DNA damage. Mol Cell 2009; 34:311 - 21; http://dx.doi.org/10.1016/j.molcel.2009.04.008; PMID: 19450529