Epigenetic regulation within stem cells
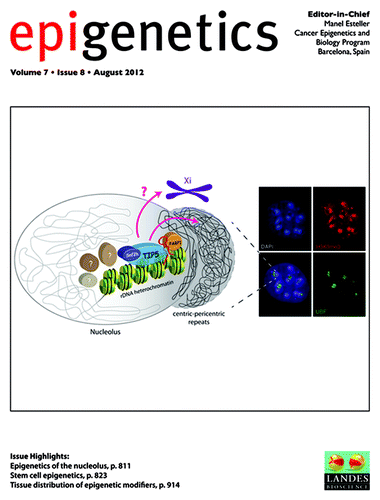
Stem cells are defined by two fundamental properties: self-renewal and pluripotency or multipotency. While until recently, the analysis of stem cells and their lineages has largely focused on transcriptional regulation, current data suggests that the genome undergoes major epigenetic alterations during embryonic stem cell (ES cell) differentiation in mammalian development. A large and complex network of epigenetic modifications governs the fine-tuning and precision of gene expression programs that define the molecular basis of stem cell pluripotency, differentiation and reprogramming. A recent review by Drs Tollervey and Lunyak summarizes the current understanding of the processes that govern this landscape in stem cells, such as histone modification, DNA methylation and alterations of chromatin structure due to chromatin remodeling and non-coding RNA activity. Further investigation into stem cell epigenetics promises to provide novel advances in the diagnosis and treatment of a wide array of human diseases ().Citation1
Loss of nuclear non-coding RNA MALAT1 is compatible with life and development
The metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) is a long non-coding RNA (lncRNA) that has been discovered as a marker for lung cancer metastasis. It is highly abundant, its expression is strongly regulated in many tumor entities including lung adenocarcinoma and hepatocellular carcinoma as well as physiological processes, and it is associated with many RNA binding proteins and highly conserved throughout evolution. The nuclear transcript MALAT1 has been functionally associated with gene regulation and alternative splicing and its regulation has been shown to impact proliferation, apoptosis, migration and invasion. In order to study the loss-of-function phenotypes of this important lncRNA, Dr Sven Diederichs and colleagues have developed a human and a mouse knockout system. In human tumor cells, MALAT1 expression was abrogated using zinc finger nucleases to stably integrate RNA destabilizing elements into the human genome site-specifically at the start of the lncRNA gene. Unexpectedly, they found that quantitative loss of MALAT1 does neither affect proliferation nor cell cycle progression nor nuclear architecture in human lung or liver cancer cells. The human loss-of-function model was complemented by a MALAT1 knockout mouse model. MALAT1-null mice showed no obvious phenotype or histological abnormalities compared with wild-type animals. The study results show that the loss of the highly abundant, nuclear enriched and evolutionarily conserved lncRNA MALAT1 is compatible with cell viability and normal development. ()Citation2
Prion formation by a yeast GLFG nucleoporin
The self-assembly of proteins into higher order structures is both central to normal biology and a dominant force in disease. Prions are self-templating protein assemblies. Originally identified as agents of infectious neurological diseases, prions are increasingly found to have diverse non-pathogenic roles. In mammals, prion-like molecular switches propagate the antiviral innate immune response; in flies they facilitate long-term memory formation; and in the budding yeast Saccharomyces cerevisiae some proteins form prions with a wide range of phenotypic consequences, suggesting that these prions function to promote phenotypic diversity and expedite adaptive evolution. Certain glutamine/asparagine (Q/N)-rich proteins in S. cerevisiae assemble into self-replicating amyloid-like protein polymers, or prions, that act as genetic elements in an entirely protein-based system of inheritance. At least seven yeast proteins are known to form prions, an additional 18 proteins contain experimentally verified prion-forming domains (PrDs), and as many as 200 have sequence characteristics of prions. Among the latter are multiple subunits of the yeast nuclear pore complex (nups; NPC). The NPC contains multiple Q/N-rich proteins whose self-assembly has also been proposed to underlie structural and functional properties of the NPC. A recent study by Dr Michael Rexach and coworkers showed that an essential sequence feature of these proteins—repeating GLFG motifs—strongly promotes their self-assembly into amyloids with characteristics of prions. Furthermore, they demonstrated that Nup100 can form bona fide prions, thus establishing a previously undiscovered ability of yeast GLFG nucleoporins to adopt this conformational state in vivo. The authors suggest that yeast has a unique capacity to exploit the aggregation tendencies of GLFG domains of nups in two ways: at the NPC, GLFG motif interactions are utilized to form a highly-effective sieve structure; whereas in the cytoplasm, they enable the formation of amyloids that can serve as benign, protein-based elements of inheritance ().Citation3
Laminopathies: Prelamin A accumulation affects BAF distribution
Tight packaging and accurate spatial organization of DNA in the nucleus is critical to gene regulation, and defects in chromatin and chromosome organization have been associated with different diseases. Recently, chromatin alterations and epigenetic changes have been identifed as common features in a group of rare genetic disorders (called laminopathies) due to mutations in the gene LMNA, encoding for two major nuclear lamina components: lamin A and lamin C. Prelamin A processing impairment is a common feature of laminopathies, and changes in histone posttranslational modifications, alterations in non-histone chromatin proteins and chromatin disorganization have been specifically linked to impairment of specific, distinct prelamin A processing steps. The molecular mechanism involved in these processes has yet to be elucidated. In a new study, Dr Christina Capanni and colleagues showed that the accumulation of wild-type prelamin A detected in restrictive dermopathy (RD), as well as the accumulation of mutated forms of prelamin A identified in familial partial lipodystrophy (FPLD) and mandibuloacral dysplasia (MADA), affects the nuclear localization of barrier-to-autointegration factor (BAF), a protein involved in lamin A-mediated chromatin remodeling functions. In accordance with previous findings, the described study supports the hypothesis of a prelamin A involvement in BAF nuclear recruitment and suggests a causative role of BAF-prelamin A complexes in prelamin A-accumulating diseases. In addition, the involvement of the inner nuclear membrane protein emerin in the proper localization of BAF-prelamin A complex was demonstrated. In conclusion, the study demonstrated that prelamin A accumulated in FPLD, MADA and RD cells interacts with BAF and affects its cellular distribution. The authors suggest that BAF-prelamin A complex may be considered as a chromatin regulator element involved in the pathophysiological mechanism of prelamin A-accumulating diseases ().Citation4
References
- Tollervey JR, Lunyak VV. Epigenetics: judge, jury and executioner of stem cell fate. Epigenetics 2012; 7:823 - 40; http://www.landesbioscience.com/journals/epigenetics/article/21141/ http://dx.doi.org/10.4161/epi.21141; PMID: 22805743
- Eißmann M, Gutschner T, Hämmerle M, Günther S, Caudron-Herger M, Groß M, et al. Loss of the abundant nuclear non-coding RNA MALAT1 is compatible with life and development. RNA Biol 2012; 9:1076 - 87 http://www.landesbioscience.com/journals/rnabiology/article/21089/
- Halfmann R, Wright JR, Alberti S, Lindquist S, Rexach M. Prion formation by a yeast GLFG nucleoporin. Prion 2012; 6:391 - 9; http://www.landesbioscience.com/journals/prion/article/20199/ http://dx.doi.org/10.4161/pri.20199; PMID: 22561191
- Capanni C, Squarzoni S, Cenni V, D’Apice MR, Gambineri A, Novelli G, et al. Familial partial lipodystrophy, mandibuloacral dysplasia and restrictive dermopathy feature barrier-to-autointegration factor (BAF) nuclear redistribution. Cell Cycle 2012; 11:3568 - 77; http://www.landesbioscience.com/journals/cc/article/21869/ http://dx.doi.org/10.4161/cc.21869; PMID: 22935701