Abstract
The passage of mRNA molecules from the site of synthesis, through the nucleoplasm and the nuclear pore, en route to the cytoplasm, might appear straightforward. Nonetheless, several decades of detailed examination of this pathway, from high resolution electron microscopy in fixed specimens, through the development of immuno-detection techniques and fluorescence toolkits, to the current era of live-cell imaging, show this to be an eventful journey. In addition to mRNAs, several species of noncoding RNAs travel and function in the nucleus, some being retained within throughout their lifetime. This review will highlight the nucleoplasmic paths taken by mRNAs and noncoding RNAs in eukaryotic cells with special focus on live-cell data and in concurrence with the biophysical nature of the nucleus.
mRNA Visualized
Visualization of mRNA in living cells requires a means of fluorescently tagging the mRNA. Tagging of endogenous mRNAs was initially performed by hybridizing fluorescent probes to mRNAs. Early on, these experiments were performed with microinjected oligo(dT) probes that would hybridize with the poly(A) tail of the mRNAs, thereby generating a system of RNA fluorescence in situ hybridization (FISH) in living cells.Citation1 Since it was not possible to differentiate by eye between the bound and the free probe, only techniques such as fluorescence correlation spectroscopy (FCS) or fluorescence recovery after photobleaching (FRAP) could be used to extract the kinetics of poly(A)+ populations in the nucleus.Citation2 Later on, as GFP became an important player in time-lapse imaging experiments, a similar but more physiological approach was taken to label mRNA. Using GFP-poly(A) binding protein (GFP-PABP) or GFP-TAP, factors that are naturally associated with all nuclear mRNAs, it was possible to examine the dynamics of the whole mRNA population without the use of hybridization.Citation3 Fluorescence recovery after photobleaching was applied to measure the movement of the GFP-tagged poly(A) RNA population, which could be differentiated from the fast moving unbound GFP-RNA-binding proteins. Since FRAP is an average-based methodology the results obtained by measuring recovery of the fluorescence in the bleached area reflect mRNAs moving at different rates.
Another approach for RNA FISH utilizes linear antisense oligodeoxynucleotides (ODNs) containing a backbone of the artificial RNA nucleotide 2' O-methyl, which is resistant to nuclease degradation.Citation4 These probes have been shown to have stronger affinity to the poly(A) tail, thus leading to a more accurate calculation of mRNPs diffusion coefficients.Citation5 This technique was further developed for use in fluorescence resonance energy transfer (FRET) experiments between two probes harboring either an acceptor or donor fluorophore.Citation6,Citation7 Using two linear probes each harboring a different fluorophore, which hybridize to adjacent sequences on a cytoplasmic mRNA, it is possible to monitor energy transfer between the fluorophores and to perform measurements to detect specific cytoplasmic mRNA FRET signals.Citation8
Additional approaches have been devised, such as: microinjection into the nucleus of in vitro synthesized and fluorescently labeled mRNAs,Citation9 complementation of split EGFP fragments fused to RNA-binding proteins that bind next to each other on the mRNA and thus reconstitute GFP fluorescence,Citation10 or an aptamer coined “Spinach” that fluoresces upon binding of GFP derivatives.Citation11 Many of these techniques have been recently discussed in detail.Citation12-Citation18 As for any scientific technology, no single approach is free of limitations. Specifically, the probe techniques typically require a rigorous method of delivery, such as microinjection, electroporation or streptolysin O permeabilization, and end with a step of hybridization that generates RNA-DNA duplexes, which is not trivial to accomplish when considering a live cell. Since mRNAs are coated with many proteins this would mean that the probes will have to compete with RNA-binding proteins and that certain portions of the RNA sequence will not be available for probe binding either due to protein binding or to secondary RNA structures. On the other hand the tagging of mRNA with GFP fusion proteins requires repeated sequences for multiple GFP-protein binding. This many times necessitates the insertion of RNA-binding sequences in tandem to be bound by many GFP-RNA binding proteins, adding exogenous protein components to the mRNP.
Many of these approaches do not enable the labeling of only a portion of the mRNA population or a specific mRNA species, not to mention the lack of single molecule sensitivity. To move the field forward it was necessary to overcome a major obstacle, namely how to efficiently tag the mRNA such that the signal-to-noise ratio is appropriate for imaging, leaving the background low enough for the detection of the mRNA molecules of interest. In a pioneer study, an oligo(dT) probe labeled with a chemically masked (caged) fluorescein molecule was used to tag the poly(A) RNA for visualization. Fluorescence of a portion of the mRNA population was achieved by irradiating the caged fluorescein using a 360 nm laser in only a small portion of the nucleus. This way the pathway of the fluorescent mRNAs could be highlighted as the un-caged signal dispersed within the nucleus, and indeed the measurements showed that mRNA motion is diffusion dominated.Citation19
Subsequently, single mRNA molecules could be followed using techniques that “light up” only the mRNA of interest and leave the background dark. For instance, molecular beacons that are administered to cells in a quenched fluorescent state and that only fluoresce once they are specifically hybridized with the mRNA.Citation20 This technique was used successfully in many cell systems.Citation17 A related approach uses hybridization of probes to cause the release of quenching of unique dyes and subsequent fluorescence.Citation21 The use of molecular beacons could be somewhat tricky due to the requirement of probe opening and restructuring prior to hybridization, whereas the use of linear antisense probes is more straightforward but does not enable single molecule detection. The latter problem was overcome by multiply labeled tetravalent RNA imaging antisense probes (MTRIPs) that have 4 or 5 fluorophores, which allowed the detection of single endogenous cytoplasmic mRNAs. This technique, however, requires the reversible permeabilization of the cells using streptolysin O.Citation22
The MS2 system, which is an RNA labeling method for the labeling of specific mRNAs, helped overcome some of the aforementioned challenges. The MS2 DNA sequence, when transcribed, forms stem-and-loop secondary structures that are recognized by an MS2 coat protein (CP) that binds as a dimer. By inserting the MS2 sequence in tandem in a gene of interest and simultaneously expressing the CP fused to a fluorescent protein, a system for tracking a specific mRNA in living cells is achieved. The repetitive nature of the MS2 sequences in the transcript, allows single molecule sensitivity by the binding of several GFP-MS2 proteins to the same mRNA. Similarly, using repetitive probes on a single mRNA sequence achieved single molecule detection.Citation23 The MS2 system usually requires the transfection of a plasmid containing the gene of interest and thereby overexpression of the mRNA, which is normally encoded by cDNA sequences lacking endogenous regulatory mRNA regions such as UTR’s and introns. Therefore, the MS2 repeats have been useful mainly in studies that stably integrate a gene of interest into the genome.Citation24-Citation31 In the case of prokaryotes or lower eukaryotes that enable relatively easy genome integration procedures, it has been possible to associate the MS2 sequence repeats with endogenous genes, as demonstrated for bacteria,Citation32,Citation33 yeast,Citation34,Citation35 Dictyostelium,Citation36,Citation37 and Drosophila.Citation38,Citation39
The overexpression issue in mammalian cells was overcome by a stable integration approach that utilizes site-specific recombination and which culminates in the expression from a single allele only.Citation40 This approach is complemented by the generation of knock-in mice containing the MS2 repeats in the 3′UTR of the endogenous β-actin gene, thereby allowing the studies of mRNA dynamics of endogenous mRNAs in the context of primary cells.Citation41
mRNA Tracked
Over the past two decades, fluorescence tagging was a key factor in the numerous attempts to unravel mRNA dynamics in the nucleus. Kinetic models applied to the data extracted from RNA FISH, FCS and FRAP experiments, enabled the calculation of mRNA diffusion coefficients and the categorization of the types of motion, such as free diffusion, corralled diffusion, or directed movement. Currently, two main approaches are applied to the study of mRNA kinetics, one being the analysis of the movement of a single mRNA particle by tracking and the other by monitoring the fluorescence recovery of a photobleached area. The former, combined with DNA labeling strategies, enabled researchers to characterize the nuclear routes of mRNA trafficking. In an early study, the nuclear distribution of the large Balbiani ring transcripts (~35kb) from Chironomus tentans was analyzed using Br-UTP labeling and immune electron microscopy. The data showed that mRNAs move randomly in between chromatin with an estimated diffusion coefficient (D) in the range of 0.08–0.12 µm2/sec. However, the nature of the recorded movement could not be decoded at the time (Brownian, corralled etc.) due to the use of fixed specimens.Citation42 In another study in mammalian cells the diffusion rates of HCMV-IE mRNA were estimated to be 0.13 µm2/sec.Citation43
The abovementioned RNA FISH techniques applied to living cells using oligo(dT)-fluorescently-labeled mRNAs, set the stage for following the movement of the poly(A) population in real-time. The first measurements in living mammalian cells, using FCS and FRAP, revealed a range of diffusion coefficients between 0.01–10 µm2/sec. One third of the diffusing oligo(dT)-poly(A) mRNAs were slow moving with average diffusion rates of < 0.59 µm2/sec.Citation2 Following that study, hybridizing a caged fluorescein oligo(dT) probe to the poly(A) RNA population and observing the dissemination of the message, supported the notion of random movement in between chromatin domains, revealing a D value of 0.6 µm2/sec for the labeled population.Citation19 A similar D (0.6 ± 0.3 µm2/sec) was found when poly(A) mRNAs were labeled with GFP-PABP2 in HeLa cells, but the data were later mathematically modeled and ended with a D value of 0.04 µm2/sec.Citation44 Upon energy depletion, a significant reduction in the movement of GFP-PABP2 was detected, implying that energy-dependent processes are involved in mRNA movement.Citation3 Since these studies differ in diffusion coefficients values, a comparison between oligo(dT) and oligo(U22)-labeled poly(A) RNAs was conducted under identical conditions using FRAP analysis. Oligo(dT) probes were found to be highly mobile due to weaker affinity to poly(A) tails, which caused discrepancies in the measurements. However, when a oligo(U22) RNA probe was used, a D value of 0.04 µm2/sec was found.Citation5
The range of diffusion coefficients obtained from poly(A) mRNA measurements suggests that different transcripts move at different rates. The development of the MS2 systemCitation24,Citation35,Citation45 and the subsequent identification of single mRNA molecules containing 24 MS2 sequence repeatsCitation45 allowed the examination of the movement of a specific mRNA molecules (mRNPs) by single particle tracking (SPT), for which a D value in the range of 0.04–0.05 µm2/sec was measured.Citation46 These measurements were corroborated by FRAP and photoactivation experiments. Diffusion governed mRNP movement and could be modeled as mostly corralled diffusion, probably due to obstruction of the pathway by chromatin domains. This strengthened the notion that mRNA movement is random. Also, even when a transcription site was observed near the nuclear membrane and in close vicinity to the NPCs, the mRNPs were seen dispersed throughout the nucleoplasm. The MS2 system was used to track specific mRNPs under ATP depletion conditions and it was found that mRNP retention due to chromatin restructuring is the reason for reduced mobility when using energy depletion treatments.Citation46 Similarly, introducing an exogenous gene with molecular beacon target sequences enabled the tracking of a specific mRNA in hamster CHO cells. Two mRNP populations were found; mobile mRNPs which had a D of 0.03 µm2/s (free diffusion) and stationary mRNPs.Citation23 The majority of studies employing the SPT methodology have used the mean square displacement model (MSD) to analyze the experimental data,Citation47 and have shown that diffusion coefficients depend on the size of the mRNA.Citation26,Citation48 However, different approaches were tested as well.Citation49 For example, jump-distance distribution analysis performed on the tracked Balbiani Ring transcript revealed 4 poly(A) RNA populations that differ in their diffusion rates. Further examination of the mRNA trajectories revealed that along their path, mRNPs sometime travel through more confined pathways, and sometime cruise through “open lanes,” concluding the same as MSD employed studies.Citation50 Interestingly, although the nucleus of C. tentans is mostly nucleolplasmic and rather devoid of chromatin, mRNPs were found to move in a discontinuous fashion, suggesting interactions and stalling at sub-nuclear structures.
Currently, the major technical difficulties in mRNA tracking are photobleaching of the fluorophore and the rapid movement in 3-dimensions. Imaging and tracking are typically performed in 2D, making it difficult to track mRNA movement from transcription site to cytoplasm. To tackle this issue, quantum dots, which are known for their fluorescence stability and strong intensity were used to track oligo(U22)-labeled poly(A) RNA in Cos7 cells for about 60 sec using 30 msec intervals (D = 0.025 µm2/sec). Although 60 sec were not enough for full pathway coverage it is a step in the right direction.Citation51
Tracking single mRNAs in the mammalian nucleus seems rather straightforward when thinking of performing this task in S. cerevisiae, since the diameter of budding yeast can be 5–6 µm, about half the diameter of a human or mouse nucleus. The yeast ARG3 housekeeping mRNA, which encodes the enzyme ornithine carbamoyltransferase, a non-localizing mRNA which is expressed at only 1–2 copies per cell, was a suitable candidate for tracking since only a few trajectories were expected thus reducing interference in a small space. The mRNA was tagged using 12 MS2 repeats integrated into the endogenous gene locus, thereby allowing expression by the endogenous promoter and the keeping of the original 3′UTR of the transcript. It was found that movement throughout the yeast cell could be categorized as random, confined or directed and diffusion coefficients were in the 0.039 µm2/sec range as observed in other tracking studies.Citation52
Single particle tracking has shown that mRNAs do not penetrate nucleoli.Citation46,Citation48 Although functionally, mRNAs are not expected to be found in the nucleolus, the question stands as to the biophysical mechanism behind this phenomenon. Single molecule analysis of fluorescent streptavidin molecules (60 kD) showed that these molecules accumulate significantly less in the nucleolus (60% less compared with the nucleoplasm), yet the nucleolar border did not impede the access of the molecules, implying that the nucleolus is a highly permeable structure to molecules of this size.Citation53 On the other hand, once inside, the movement was highly mobile. This was explained by measurements of nucleoplasmic vs. nucleolar density showing that there is substantially less free space in the nucleolus, therefore excluding molecules that do not have binding sites within the nucleolus. Nevertheless, streptavidin molecules are rather small compared with mRNP complexes and indeed exclusion of dextran molecules from the nucleolus has been shown to be size-dependent, as well as causing a significant reduction in diffusion in this dense compartment.Citation54 The exclusion of mRNPs from nucleoli was directly visualized as the actual “bumping off” of single mRNPs from nucleoli in live-cell movies.Citation46 Moreover, during osmotic treatment nucleolar regions became completely accessible to mRNPs.Citation48
mRNP molecules appear as rounded particles in time-lapse fluorescence microscopy movies, yet it is hard to infer structure from these images due to the resolution of light microcopy. Visualization of RNPs by electron microscopy indeed shows that these are compact and moderately elongated structures,Citation55-Citation57 and thereby can be regarded as particulate structures in analyses that model mRNP dynamics.Citation58 A simulation of mRNA diffusion in the nucleus has shown that even if the mRNA wanders around the nucleus for a long time, it will find its way out.Citation59 Also, regions of high density where an mRNA would have low diffusion coefficients would “push” mRNA toward the inter-chromatin domain that due to its small volume reduces the space in which an mRNA has to search until it finds an NPC. Altogether, this leads to efficient exploration of the inter-chromatin space and to rapid exit times.
RNAs Stuck in the Nucleus
mRNAs are an easy target for tracking in living cells due to their size and our knowledge of the functional outcome inherent in them. Tracking of small RNAs, which are typically complexed with proteins to form small nuclear ribonucleoprotein particles, has been more challenging.Citation60 For instance, fluorescently labeled U1 snRNPs, which are part of the core splicing machinery, were tracked at single molecule resolution in the mammalian nucleus and were shown to have diffusion coefficients in the range of 0.5–8 µm2/sec. The snRNPs were observed to transiently bind to immobile sites with short dwell times (50–350 msec), suggesting the detection of snRNPs involved in active splicing.Citation61,Citation62 The diffusion coefficient of fluorescein-tagged U7 snRNA, was measured in the nucleoplasm of Xenopus oocytes and found to be 0.26 µm2/sec.Citation63 The behavior of snRNPs in nuclei of living cells was further elucidated using GFP-tagged splicing factors expressed at either exogenousCitation64,Citation65 or endogenousCitation66 levels and following their kinetics using FCS and FRAP analysis.Citation67 Further to obtaining diffusion coefficients for the different snRNP species, this studyCitation67 showed that U1 and U4/U6 snRNP components have a dwell time of less than 1 sec, whereas U2 and U5 snRNP have longer interactions in the range of 15–30 sec. Altogether, these results allow to estimate that the time-frame of the splicing reaction is around 30 sec,Citation67-Citation70 although also longer times (0.5 min and 10 min) have been estimated.Citation71,Citation72 The mobility of snRNPs was found to be higher in nuclei of cells from spinal muscle atrophy (SMA) patients, a genetic disease that is caused due to low levels of the survival motor neuron (SMN) protein. This implies a reduction in the interactions of snRNPs with their nuclear binding sites and less incorporation into active spliceosomes, possibly leading to the wide-spread splicing defects associated with this disease.Citation73
Another example of a small nuclear retained RNA is found in the telomerase enzyme that functions in maintaining the length of telomere arms of linear eukaryotic chromosomes. Telomerase is comprised of several proteins and an RNA component, the latter serving as a template for the addition of telomere DNA sequences. Tagging of the yeast RNA component called TLC1 RNA with MS2 repeats enabled the tracking of telomerase in real-time.Citation74 Contrary to the assumption that telomerase is stably associated with the telomeres, tracking of the telomerase particles in living cells showed a diffusive behavior similar to mRNPs.Citation46 Following these dynamics throughout the cell cycle led to an interesting observation. During late S phase telomerase particles were larger and brighter than observed in G1 or G2 and the dynamics of these clusters correlated with the dynamics of the telomeric ends, implying a temporary stable association between telomeres and telomerase. Indeed, these structures were found to represent elongating telomerase complexes.
The expansion of CTG trinucleotide repeats in the 3′UTR of the dystrophia myotonica protein kinase (DMPK) gene causes myotonic dystrophy type I. Nuclear retention of the mutant mRNA is caused by the long 3′UTR CUG tract and is observed as mRNA aggregates localized in nuclear foci. Supposedly, these foci disrupt normal cellular function by sequestering important factors. Since these foci are observed in proximity to nuclear speckles it has been suggested that because the DMPK mRNAs are unable to enter speckles, they cannot complete the pathway toward export.Citation75,Citation76 Using a transcript containing the MS2 sequence repeats and a stretch of 145 CUG repeats, it was possible to bring about the aggregation of some of these mRNAs in nuclear foci whereas the remainder of the mRNAs were nuclear retained. The 145x-CUG mRNPs were tracked and showed significantly slower diffusion rates compared with mRNAs that contained only 5 CUG repeats (145x-CUG: 0.025 µm2/sec for diffusive mRNPs and 0.006 µm2/sec for corralled mRNPs; 5x-CUG: 0.054 µm2/sec for diffusive mRNPs and 0.0043 µm2/sec for corralled mRNPs).Citation77 The diffusion coefficients for the 5-CUG mRNPs fall in the same range as measured for nuclear single mRNPs,Citation5,Citation23,Citation44,Citation46 whereas the slightly larger 145x-CUG single mRNPs and the nuclear aggregates had considerably reduced and restricted nuclear mobility.
Since the size of the 145x-CUG mRNA is not significantly larger than the 5x-CUG mRNA, the reduction in diffusion coefficients cannot be attributed to mRNP size, as previously shown for large dystrophin mRNPs,Citation48 and therefore another possibility could then be a reduction in mobility due to binding which would hinder the movement. Indeed, FRAP and fluorescence loss in photobleaching (FLIP) analyses of the nuclear aggregates showed that mRNPs within these structures were in consistent exchange with the nucleoplasm and were undergoing aggregation-disaggregation cycles. Moreover, the mRNA nuclear retention effect was not attributed to the aggregation phenomenon but rather to the presence of a splicing factor, Mbnl1, within the nuclear foci. Knockdown of Mbnl1 led to an increase in the translation of the protein product of the 145-CUG mRNA without any change in the mRNA levels, thus implying that the factor directly affects mRNA export of the expanded mRNAs. Interestingly, these mRNAs are spliced prior to their arrival at the nuclear aggregatesCitation76 suggesting that the role of Mnbl1 in the nuclear retention effect is not directly related to the splicing of the CUG transcripts.
mRNA Exported
Pulse chase labeling of nascent mRNA with bromo-uridine (Br-U) and subsequent quantification by electron microscopy (EM) showed that within a time-frame of 50 min the sites of transcription had cleared, and after traveling through the nucleoplasm as 200S particles, reminiscent of the supra-spliceosome structures,Citation78 the mRNAs were found attached 200 nm away from the pore, most likely denoting the attachment to the nuclear basket. Little mRNA was seen inside NPCs suggesting that export is very rapid.Citation79,Citation80
Poly(A) mRNAs detected in EM sections of mammalian cells by RNA FISH could be observed protruding through the nuclear pore and accumulating on the cytoplasmic side. Examination of cross-sections showed that all NPCs were stained and therefore capable of exporting mRNA,Citation81 as also seen initially in XenopusCitation82 and in Br-U labeling experiments in HeLa cells.Citation79 One study has shown differential staining of NPCs for either poly(A) RNA or for NTF2 that facilitates protein transport.Citation83 Still, as the NPC channel is considered a crowded environment, and the bi-directional flux of cargo is significant,Citation84,Citation85 there should be considerable competition for binding sites within the NPC. Therefore, this finding might reflect a given moment rather than the specialization of NPCs for the transfer of specific cargoes.
Where within the NPC do mRNAs translocate? Intriguingly, EM staining of various cargoes has delineated two pathways of cargo movement within the NPC—a central path vs. a peripheral path (). This demarcation has been recently modeled in vitro, showing a central hydrophobic and electrostatically positive region that could act as an electrostatic barrier to positively charged cargoes and as an attractant of negatively charged macromolecules with hydrophobic surfaces. A second more peripheral zone contains positively charged amino acid residues thereby attributing the passageway with a hydrophilic character.Citation86 As for mRNA, RNA FISH in human cells showed that the staining was more intense in the pore periphery than in the middle,Citation81 whereas gold labeling of mRNA showed central channel localization in Xenopus nuclear pores,Citation80 as also observed in C. tentans.Citation87,Citation88 High resolution analysis of this issue in human hematopoietic HL-60 cells and in rat liver tissue using Br-U labeled RNA and EM, as well as electron spectroscopic imaging (ESI), showed that mRNAs traffic via the periphery of the pore rather than through the central axis.Citation83 In a study that determined the positions different cargo undertake while passing through the pore of S. cerevisiae, the mRNA export factors Dbp5 and its partner Gle1 were centrally localized, thus suggesting that the later stages of mRNA export might be centrally located.Citation89
Figure 1. Following the pathway of mRNA in the nucleus. (A) mRNA labeling and tagging. Four main mRNA tagging methods are depicted in green: (1) Microinjection of in vitro synthesized and labeled mRNAs; (2) Hybridization of fluorescently-tagged DNA/RNA probes or molecular beacons (MB); (3) GFP fused-RNA binding proteins (GFP-PABP2 and GFP-TAP); and (4) MS2 tagging with the MS2-coat protein (CP). (B) mRNA tracking. Current methodologies for mRNA kinetic studies: single particle tracking (SPT), fluorescence correlation spectroscopy (FCS) and fluorescence recovery after photobleaching (FRAP). In yellow are the point of detection for FCS and the region photobleached during FRAP, and arrows mark the movement in and out of the spots. Fluorescent mRNPs are in green and photobleached ones are in black. (C) mRNA export. Left: The region in the pore through which mRNA (black dots) translocation occurs is represented by two paths (peripheral and central), as observed in EM studies. Right: the kinetic time-frame of mRNA movement through the nucleus and through the pore is described in C. tentans and mammalian cells. Chromatin regions appears in blue.
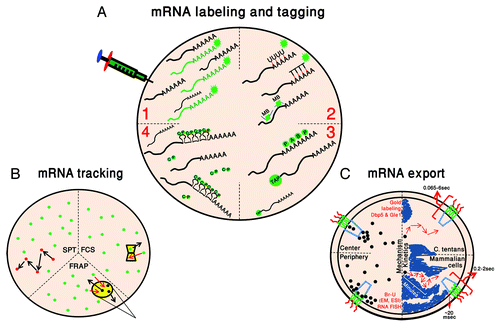
While EM experiments of mRNA staining have arrived at the conclusion that mRNA passage through the NPC must be very rapid, the time dimension was missing from these studies,Citation90 and only recently was this issue tackled and demonstrated in living cells. Tracking large mRNAs expressed from exogenous genes in human cells showed the rather slow diffusion of mRNPs through the nucleoplasm (time-scale of minutes), compared to the speedy exit from the nucleus on the range of less than 500 msec.Citation48 High-resolution tracking of endogenous β-actin mRNAs in mouse cells has provided a breakdown of the timeframes for mRNP docking to the NPC and mRNP translocation through the pore and has found that the passage time of ~200 msec is spent mostly at the docking site at the nuclear basket and on the cytoplasmic filaments (although there was a subpopulation of the transcripts that spent ~2 sec at the pore), whereas the dwell time in the pore was around 20 msec.Citation91 This study suggests that there are unique docking and release steps for an exported mRNP. Recently, the export of the Balbiani ring (BR) mRNPs in living cells has been observed. The BR mRNP of C. tentans has served as a primary model for mRNA export due to its large size (32–40 kb) and thereby its detectability in EM sections. Seminal studies on native BR mRNPs led to the understanding that the mRNA export process is directional since the 5′ of the mRNA was observed to protrude first on the cytoplasmic side of the NPC. Moreover, in order to physically pass through the pore, the large mRNPs had to undergo a certain degree of unfolding or restructuring.Citation88,Citation92-Citation95 Following BR mRNPs in live C. tentans salivary gland cells by use of microinjected fluorescently-labeled hrp36, the homolog of mammalian hnRNP A1, and rapid imaging of the mRNPs with light-sheet fluorescence microscopy, demonstrated that export times could range between 65 msec and 6 sec.Citation96 This analysis could also detect different modes of interaction: short interactions with the nuclear envelope suggested short-lived collisions with the NPCs, longer interactions that would agree with unsuccessful export events, and a relatively long binding step to the nuclear basket prior to effective export, which suggests that there is a rate-limiting step at the nuclear basket. Indeed, blocking of mRNA export in mammalian cells using a mutant form of the Dbp5 DEAD-box helicase supposedly required for release of export factors and restructuring of the mRNA during export, caused the docking step of mRNPs to be detected, as mRNPs remained bound to the nuclear envelope for long time periods due to the block,Citation97 whereas such an occurrence is not typically observed due to the rapid kinetics of export.
Nuclear Structure and Dynamics Come Together
Chromatin regions, whether dense or open, have been shown to be relatively accessible to protein factors (even large ones) involved in replication, transcription and RNA processing. This was demonstrated by following the sub-nuclear distribution of microinjected, different-sized, fluorescent dextrans and large GFP-fusions (~550–600 kD) in mammalian cell nucleiCitation98 and in agreement with an earlier study that demonstrated that large proteins (~500 kD) can rapidly diffuse in the cytoplasm and the nucleus.Citation99
Dense chromatin regions and nucleoli obstruct the movement of large structures such as mRNPs in the nucleus, and therefore mRNA diffusion can occur in nucleoplasmic space typically referred to as the inter-chromatin compartmentCitation100 or extra-chromosomal channel network.Citation101 Electron microscopy studies have provided high resolution information as to where mRNA and DNA domains are in the nucleus.Citation102,Citation103 Newly synthesized transcripts are found on the border between compact chromatin domains and the inter-chromatin space, in a region termed the peri-chromatin region (PR). RNA polymerase II, transcription factors and RNA processing factors are found to localize to this region as well. In situ hybridization with RNA probes to poly(A) mRNAs, rather than DNA probes, on ultrathin sections were examined by electron microscopy and ultimately showed that the peri-chromatin region not only contains the transcribed mRNAs but also harbors the transcribed DNA regions,Citation104 and might reflect transcription factories.Citation105 mRNAs could also be observed in the inter-chromatin space.
A limited area for pathway of mRNAs traveling in the nucleus was suggested by several studies. RNA FISH to poly(A) mRNA observed by EM showed mRNAs localized in confined regions approaching the NPCsCitation81 and in extra-chromosomal channels in the Drosophila nucleus.Citation101 Accumulations of mRNAs in restricted pathways were coined mRNA tracks and have been observed for several types of mammalian and viral mRNA species.Citation106-Citation113 Interestingly, the structural integrity of the terminal inter-chromatin pathways, which are observed by EM leading up to the NPCs and bordered by peripheral heterochromatin regions to form a cone-like zone at the NPC, is dependent on the nuclear basket protein Tpr.Citation114 Knockdown of Tpr resulted in the formation of an abnormal layer of condensed chromatin all around the nuclear envelope, thus probably blocking transport through NPCs. The clear demarcation between the DNA and RNA compartments was finally visualized in living cells by the abovementioned use of photoactivation of caged oligo(dT) probes that hybridized with poly(A) mRNAs,Citation19 showing a network of paths in which poly(A) mRNAs translocate in between distinct DNA domains. This finding was enhanced by showing the movement of single mRNPs in the nuclear space that lies in between dense chromatin regions.Citation48 The RNAs moved in a compartment that included nuclear speckles, Cajal bodies and PML bodies,Citation46,Citation107 and could be clearly observed during ATP depletion experiments that caused the condensation of the chromatin and its segregation from the nucleoplasmCitation46 and similarly during osmotic treatment.Citation115
Transient and reversible chromatin compaction caused by hyper-osmotic conditions, that also leads to an enlargement of the inter-chromatin space, shows that the transcribing DNA regions remain located in the PR despite the structural change in chromatin, implying that even during chromatin condensation the active DNA regions remain in the periphery of the chromatin domain.Citation104 The authors of this study, in which mRNA granules were detected mostly in the PR, suggest the mRNPs travel along the PR regions all the way to the NPC. These observations in cell sections might correlate to the corralled movements of mRNAs seen by single mRNA tracking, that were thought to reflect interaction or stalling by chromatin regions.Citation46 Simulation of mRNA diffusion in the nucleus has shown that mRNAs will spend most of their time close to the interface between the chromatin and the inter-chromatin and that if mRNAs wander deep into the chromatin, they get stuck there for long time periods.Citation59 Since several processes examine mRNP quality to verify that only a competent mRNP leaves the nucleus and act from the time of transcription and at the NPC,Citation116 it is possible that the stalling events during trafficking might also reflect binding by quality control factors. Indeed, we know that specific factors associate with the mRNA and facilitate export, such as Aly/REF, TAP/NXF1/MEX67, GANP or TREX export factors,Citation117-Citation122 or the exon-junction-complex (EJC) proteins that accumulate following pre-mRNA splicing.Citation123,Citation124 Furthermore, it has been suggested that protein composition could change along the nucleoplasmic route.Citation125 However, much of the spatial and temporal details of these processes remain elusive. It stands to reason that quality control is a multi-step process since the recruitment of Tap/NXF1 aloneCitation126 or the mere splicing of an mRNACitation127 are not sufficient for fruitful mRNA export.
Biophysics of the Nucleus
Assembling the different pieces of the puzzle together, we are now able to appreciate the emerging picture of nuclear biophysics. Studies with fluorescent dextrans showed that diffusion in the nucleus is several fold slower than in water.Citation99,Citation128,Citation129 Still, proteins move through the cell at high rates, as seen from bacteria to mammalian cells.Citation130,Citation131 Hence, the nucleus is viewed as an aqueous but crowded environment due to the high abundance of molecules, macromolecules and large complexes such as mRNPs and pre-ribosomal subunits.Citation132 Studies have shown that osmotic extraction of water from cells leads to the reversible compaction of chromatin and to segregation of the chromatin domain and the nucleoplasmic domain.Citation115,Citation133
The diffusion of molecules or complexes within the nucleus will be hindered by obstacles such as chromatin. Still, molecules in the nucleus must reach a certain destination, whether it is a transcription factor binding to a specific promoter sequence, a splicing factor attaching to a specific exon-intron junction in the pre-mRNA, or a mature mRNA traveling from the gene to the NPC. Since diffusion underlies the motion of molecules in the nucleus, it is supposed that the landscape of the nucleus, defined by the density of chromatin and nuclear bodies within, allows for the regulation of flow and the retention of molecules at specific sites of biological significance. A recent study using magnetic beads microinjected into the nucleus and followed at either 25°C or 37°C with or without magnetic force, has shown that the active remodeling of chromatin by ATP-dependent chromatin remodeling enzymes may drive molecule currents in the nucleoplasm.Citation134 Another study using FCS to analyze the movement of EGFP, an inert molecule, throughout the nucleus established a “diffusion map.”Citation135 Using histone labeling by H2B-mRFP, the density of chromatin regions could be used to compare EGFP diffusion in different compartments. EGFP was homogenously distributed and diffusion was found to be similar throughout the nucleus without any impediment by the chromatin network. Consequently, the actual traveling times of EGFP could be measured between two locations,Citation136 in contrast to FCS that provides measurement of the molecules at one spot only without information of the pathway the molecule takes before or after entering the measurement spot. This analysis was performed with pair correlation function analysis (pCF) that measures anistotropic diffusion of EGFP molecules and showed that two types of flow could be detected that are influenced by the density of DNA in the region (density was measured using Hoechst 33342 DNA labeling). Because of the different DNA densities, a channel-like network appears in which EGFP can freely diffuse. However, flow or movement between the two compartments of either high or low density is restricted. Comparison to fluorescein, a much smaller molecule than EGFP, showed that diffusion of a small molecule was not obstructed in any way by high density chromatin. Interestingly, an infrequent burst-like movement of EGFP through the barrier between high/low density regions was also observed. This is a ~300 msec occurrence that suggests chromatin restructuring has taken place. Altogether, this approach demonstrates the organization of a channeled network of chromatin thereby directing molecules between different compartments through differences in DNA density.
Expanding Our Vision of RNA Dynamics
As we delve deeper into the nuclear microenvironment, we find it is now possible to finally face old scientific problems with new technologies and the broadened perspective of nuclear processes. For instance, we long to understand how the large mRNA-protein complexes manage to travel efficiently through the NPC. While export is a rather fast processCitation90,Citation91,Citation96 large mRNPs may take longer due to size and the need for restructuring, as shown for the BR mRNP that undergoes structural changes as it squeezes through the NPC,Citation93 or for large mammalian mRNPs.Citation90 Noteworthy regarding mRNA export, it has been suggested that the newly identified export pathway that does not use the NPCCitation137 might be necessary for large cargos that do not pass through the NPC,Citation138 and also resembles the egress of herpesvirus capsids from the nucleus.Citation139 While we tend to think of RNA movement from nucleus to cytoplasm as a one way road,Citation140 we should consider that the reverse is possible as well and might be physiologically relevant.Citation91,Citation141,Citation142 Moreover, we know that cytoplasmic mechanics can affect gene expression patterns in the nucleus by transducing forces via the proteins spanning the nuclear envelope.Citation143-Citation145 These forces might reflect on RNA dynamics too, as shown for nuclear body proteins.Citation146
The dynamic properties of non-coding RNAs still await in-depth investigation, as is being performed for mRNA. While mRNAs typically display nucleo-cytoplasmic trafficking as a default mechanism, many non-coding RNAs are either nuclear retained or require a unique signal in order to facilitate translocation to the cytoplasm.Citation147-Citation149 Similarly, viral RNAs and their lifecycle also deserve renewed attention. For example, the polyadenylated nuclear (PAN) non-coding RNA is transcribed by RNA Pol II and is highly expressed during the lytic phase of Kaposi sarcoma-associated herpesvirus (KSHV) infection, during which its remains nuclear localized. Interestingly, during lytic KSHV infection the cytoplasmic poly(A)-binding protein C1 (PABPC1) relocalizes to the nucleus where it binds PAN RNA.Citation150,Citation151 In the case of PAN RNA, this RNA overloads the nucleus (500,000 transcript/nucleus), outnumbering by far the host and viral RNAs, as this species comprises ~80% of all polyadenylated RNAs during lytic infection. It remains to be seen what the function is of many of the coding and non-coding RNAs of viruses and how these affect the nuclear dynamics of host mRNAs either by direct interactions, titrating of RNA-binding proteins or overloading of the nucleus.
Recently, another mRNA tagging system based on repetitive sequences has been implemented in mRNA quantification and live-cell imaging experiments. Similar to the MS2 system, the PP7 tag is based on an RNA hairpin sequence and its cognate binding protein from the Pseudomonas aeruginosa PP7 bacteriophage.Citation152 Currently, this tag has been used together with the MS2 tag in yeast cells and has delivered new insights into the kinetics of transcription and RNA localization in yeast, plant and mammalian cells.Citation153-Citation157 Further utilization of the PP7 system, which might be even better than the MS2 system in mammalian cells,Citation158 will be worthwhile for broadening our capacity to visualize and correlate between two mRNA species in the same cell.
| Abbreviations: | ||
| BR | = | Balbiani ring |
| EM | = | electron microscopy |
| FCS | = | fluorescence correlation spectroscopy |
| FISH | = | fluorescence in situ hybridization |
| FLIP | = | fluorescence loss in photobleaching |
| FRAP | = | fluorescence recovery after photobleaching |
| FRET | = | fluorescence resonance energy transfer |
| NPC | = | nuclear pore complex |
| ODN | = | oligodeoxynucleotide |
| PABP | = | poly(A) binding protein |
Acknowledgments
Yaron Shav-Tal is supported by the European Research Council (ERC).
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
References
- Politz JC, Taneja KL, Singer RH. Characterization of hybridization between synthetic oligodeoxynucleotides and RNA in living cells. Nucleic Acids Res 1995; 23:4946 - 53; http://dx.doi.org/10.1093/nar/23.24.4946; PMID: 8559650
- Politz JC, Browne ES, Wolf DE, Pederson T. Intranuclear diffusion and hybridization state of oligonucleotides measured by fluorescence correlation spectroscopy in living cells. Proc Natl Acad Sci U S A 1998; 95:6043 - 8; http://dx.doi.org/10.1073/pnas.95.11.6043; PMID: 9600914
- Calapez A, Pereira HM, Calado A, Braga J, Rino J, Carvalho C, et al. The intranuclear mobility of messenger RNA binding proteins is ATP dependent and temperature sensitive. J Cell Biol 2002; 159:795 - 805; http://dx.doi.org/10.1083/jcb.200203046; PMID: 12473688
- Molenaar C, Marras SA, Slats JC, Truffert JC, Lemaître M, Raap AK, et al. Linear 2′ O-Methyl RNA probes for the visualization of RNA in living cells. Nucleic Acids Res 2001; 29:E89 - 9; http://dx.doi.org/10.1093/nar/29.17.e89; PMID: 11522845
- Molenaar C, Abdulle A, Gena A, Tanke HJ, Dirks RW. Poly(A)+ RNAs roam the cell nucleus and pass through speckle domains in transcriptionally active and inactive cells. J Cell Biol 2004; 165:191 - 202; http://dx.doi.org/10.1083/jcb.200310139; PMID: 15117966
- Tsuji A, Koshimoto H, Sato Y, Hirano M, Sei-Iida Y, Kondo S, et al. Direct observation of specific messenger RNA in a single living cell under a fluorescence microscope. Biophys J 2000; 78:3260 - 74; http://dx.doi.org/10.1016/S0006-3495(00)76862-7; PMID: 10828002
- Tsuji A, Sato Y, Hirano M, Suga T, Koshimoto H, Taguchi T, et al. Development of a time-resolved fluorometric method for observing hybridization in living cells using fluorescence resonance energy transfer. Biophys J 2001; 81:501 - 15; http://dx.doi.org/10.1016/S0006-3495(01)75717-7; PMID: 11423432
- Okabe K, Harada Y, Zhang J, Tadakuma H, Tani T, Funatsu T. Real time monitoring of endogenous cytoplasmic mRNA using linear antisense 2′-O-methyl RNA probes in living cells. Nucleic Acids Res 2011; 39:e20; http://dx.doi.org/10.1093/nar/gkq1196; PMID: 21106497
- Tadakuma H, Ishihama Y, Shibuya T, Tani T, Funatsu T. Imaging of single mRNA molecules moving within a living cell nucleus. Biochem Biophys Res Commun 2006; 344:772 - 9; http://dx.doi.org/10.1016/j.bbrc.2006.03.202; PMID: 16631111
- Ozawa T, Natori Y, Sato M, Umezawa Y. Imaging dynamics of endogenous mitochondrial RNA in single living cells. Nat Methods 2007; 4:413 - 9; PMID: 17401370
- Paige JS, Wu KY, Jaffrey SR. RNA mimics of green fluorescent protein. Science 2011; 333:642 - 6; http://dx.doi.org/10.1126/science.1207339; PMID: 21798953
- Santangelo PJ, Alonas E, Jung J, Lifland AW, Zurla C. Probes for intracellular RNA imaging in live cells. Methods Enzymol 2012; 505:383 - 99; http://dx.doi.org/10.1016/B978-0-12-388448-0.00028-0; PMID: 22289464
- Bann DV, Parent LJ. Application of live-cell RNA imaging techniques to the study of retroviral RNA trafficking. Viruses 2012; 4:963 - 79; http://dx.doi.org/10.3390/v4060963; PMID: 22816035
- Armitage BA. Imaging of RNA in live cells. Curr Opin Chem Biol 2011; 15:806 - 12; http://dx.doi.org/10.1016/j.cbpa.2011.10.006; PMID: 22055496
- Urbinati CR, Long RM. Techniques for following the movement of single RNAs in living cells. Wiley Interdiscip Rev RNA 2011; 2:601 - 9; http://dx.doi.org/10.1002/wrna.83; PMID: 21957047
- Christensen NM, Oparka KJ, Tilsner J. Advances in imaging RNA in plants. Trends Plant Sci 2010; 15:196 - 203; http://dx.doi.org/10.1016/j.tplants.2010.01.005; PMID: 20153241
- Tyagi S. Imaging intracellular RNA distribution and dynamics in living cells. Nat Methods 2009; 6:331 - 8; http://dx.doi.org/10.1038/nmeth.1321; PMID: 19404252
- Dictenberg J. Genetic encoding of fluorescent RNA ensures a bright future for visualizing nucleic acid dynamics. Trends Biotechnol 2012; 30:621 - 6; http://dx.doi.org/10.1016/j.tibtech.2012.09.004; PMID: 23127753
- Politz JC, Tuft RA, Pederson T, Singer RH. Movement of nuclear poly(A) RNA throughout the interchromatin space in living cells. Curr Biol 1999; 9:285 - 91; http://dx.doi.org/10.1016/S0960-9822(99)80136-5; PMID: 10209094
- Bratu DP, Cha BJ, Mhlanga MM, Kramer FR, Tyagi S. Visualizing the distribution and transport of mRNAs in living cells. Proc Natl Acad Sci U S A 2003; 100:13308 - 13; http://dx.doi.org/10.1073/pnas.2233244100; PMID: 14583593
- Kubota T, Ikeda S, Yanagisawa H, Yuki M, Okamoto A. Sets of RNA repeated tags and hybridization-sensitive fluorescent probes for distinct images of RNA in a living cell. PLoS One 2010; 5:e13003; http://dx.doi.org/10.1371/journal.pone.0013003; PMID: 20885944
- Santangelo PJ, Lifland AW, Curt P, Sasaki Y, Bassell GJ, Lindquist ME, et al. Single molecule-sensitive probes for imaging RNA in live cells. Nat Methods 2009; 6:347 - 9; http://dx.doi.org/10.1038/nmeth.1316; PMID: 19349979
- Vargas DY, Raj A, Marras SA, Kramer FR, Tyagi S. Mechanism of mRNA transport in the nucleus. Proc Natl Acad Sci U S A 2005; 102:17008 - 13; http://dx.doi.org/10.1073/pnas.0505580102; PMID: 16284251
- Janicki SM, Tsukamoto T, Salghetti SE, Tansey WP, Sachidanandam R, Prasanth KV, et al. From silencing to gene expression: real-time analysis in single cells. Cell 2004; 116:683 - 98; http://dx.doi.org/10.1016/S0092-8674(04)00171-0; PMID: 15006351
- Darzacq X, Shav-Tal Y, de Turris V, Brody Y, Shenoy SM, Phair RD, et al. In vivo dynamics of RNA polymerase II transcription. Nat Struct Mol Biol 2007; 14:796 - 806; http://dx.doi.org/10.1038/nsmb1280; PMID: 17676063
- Ben-Ari Y, Brody Y, Kinor N, Mor A, Tsukamoto T, Spector DL, et al. The life of an mRNA in space and time. J Cell Sci 2010; 123:1761 - 74; http://dx.doi.org/10.1242/jcs.062638; PMID: 20427315
- Brody Y, Neufeld N, Bieberstein N, Causse SZ, Böhnlein EM, Neugebauer KM, et al. The in vivo kinetics of RNA polymerase II elongation during co-transcriptional splicing. PLoS Biol 2011; 9:e1000573; http://dx.doi.org/10.1371/journal.pbio.1000573; PMID: 21264352
- Boireau S, Maiuri P, Basyuk E, de la Mata M, Knezevich A, Pradet-Balade B, et al. The transcriptional cycle of HIV-1 in real-time and live cells. J Cell Biol 2007; 179:291 - 304; http://dx.doi.org/10.1083/jcb.200706018; PMID: 17954611
- Shanbhag NM, Rafalska-Metcalf IU, Balane-Bolivar C, Janicki SM, Greenberg RA. ATM-dependent chromatin changes silence transcription in cis to DNA double-strand breaks. Cell 2010; 141:970 - 81; http://dx.doi.org/10.1016/j.cell.2010.04.038; PMID: 20550933
- Martins SB, Rino J, Carvalho T, Carvalho C, Yoshida M, Klose JM, et al. Spliceosome assembly is coupled to RNA polymerase II dynamics at the 3′ end of human genes. Nat Struct Mol Biol 2011; 18:1115 - 23; http://dx.doi.org/10.1038/nsmb.2124; PMID: 21892168
- de Turris V, Nicholson P, Orozco RZ, Singer RH, Mühlemann O. Cotranscriptional effect of a premature termination codon revealed by live-cell imaging. RNA 2011; 17:2094 - 107; http://dx.doi.org/10.1261/rna.02918111; PMID: 22028363
- Golding I, Cox EC. RNA dynamics in live Escherichia coli cells. Proc Natl Acad Sci U S A 2004; 101:11310 - 5; http://dx.doi.org/10.1073/pnas.0404443101; PMID: 15277674
- Golding I, Paulsson J, Zawilski SM, Cox EC. Real-time kinetics of gene activity in individual bacteria. Cell 2005; 123:1025 - 36; http://dx.doi.org/10.1016/j.cell.2005.09.031; PMID: 16360033
- Haim-Vilmovsky L, Gadir N, Herbst RH, Gerst JE. A genomic integration method for the simultaneous visualization of endogenous mRNAs and their translation products in living yeast. RNA 2011; 17:2249 - 55; http://dx.doi.org/10.1261/rna.029637.111; PMID: 22025736
- Bertrand E, Chartrand P, Schaefer M, Shenoy SM, Singer RH, Long RM. Localization of ASH1 mRNA particles in living yeast. Mol Cell 1998; 2:437 - 45; http://dx.doi.org/10.1016/S1097-2765(00)80143-4; PMID: 9809065
- Chubb JR, Trcek T, Shenoy SM, Singer RH. Transcriptional pulsing of a developmental gene. Curr Biol 2006; 16:1018 - 25; http://dx.doi.org/10.1016/j.cub.2006.03.092; PMID: 16713960
- Muramoto T, Cannon D, Gierlinski M, Corrigan A, Barton GJ, Chubb JR. Live imaging of nascent RNA dynamics reveals distinct types of transcriptional pulse regulation. Proc Natl Acad Sci U S A 2012; 109:7350 - 5; http://dx.doi.org/10.1073/pnas.1117603109; PMID: 22529358
- Forrest KM, Gavis ER. Live imaging of endogenous RNA reveals a diffusion and entrapment mechanism for nanos mRNA localization in Drosophila. Curr Biol 2003; 13:1159 - 68; http://dx.doi.org/10.1016/S0960-9822(03)00451-2; PMID: 12867026
- Zimyanin VL, Belaya K, Pecreaux J, Gilchrist MJ, Clark A, Davis I, et al. In vivo imaging of oskar mRNA transport reveals the mechanism of posterior localization. Cell 2008; 134:843 - 53; http://dx.doi.org/10.1016/j.cell.2008.06.053; PMID: 18775316
- Yunger S, Rosenfeld L, Garini Y, Shav-Tal Y. Single-allele analysis of transcription kinetics in living mammalian cells. Nat Methods 2010; 7:631 - 3; http://dx.doi.org/10.1038/nmeth.1482; PMID: 20639867
- Lionnet T, Czaplinski K, Darzacq X, Shav-Tal Y, Wells AL, Chao JA, et al. A transgenic mouse for in vivo detection of endogenous labeled mRNA. Nat Methods 2011; 8:165 - 70; http://dx.doi.org/10.1038/nmeth.1551; PMID: 21240280
- Singh OP, Björkroth B, Masich S, Wieslander L, Daneholt B. The intranuclear movement of Balbiani ring premessenger ribonucleoprotein particles. Exp Cell Res 1999; 251:135 - 46; http://dx.doi.org/10.1006/excr.1999.4490; PMID: 10438579
- Snaar SP, Verdijk P, Tanke HJ, Dirks RW. Kinetics of HCMV immediate early mRNA expression in stably transfected fibroblasts. J Cell Sci 2002; 115:321 - 8; PMID: 11839784
- Braga J, McNally JG, Carmo-Fonseca M. A reaction-diffusion model to study RNA motion by quantitative fluorescence recovery after photobleaching. Biophys J 2007; 92:2694 - 703; http://dx.doi.org/10.1529/biophysj.106.096693; PMID: 17259280
- Fusco D, Accornero N, Lavoie B, Shenoy SM, Blanchard JM, Singer RH, et al. Single mRNA molecules demonstrate probabilistic movement in living mammalian cells. Curr Biol 2003; 13:161 - 7; http://dx.doi.org/10.1016/S0960-9822(02)01436-7; PMID: 12546792
- Shav-Tal Y, Darzacq X, Shenoy SM, Fusco D, Janicki SM, Spector DL, et al. Dynamics of single mRNPs in nuclei of living cells. Science 2004; 304:1797 - 800; http://dx.doi.org/10.1126/science.1099754; PMID: 15205532
- Saxton MJ, Jacobson K. Single-particle tracking: applications to membrane dynamics. Annu Rev Biophys Biomol Struct 1997; 26:373 - 99; http://dx.doi.org/10.1146/annurev.biophys.26.1.373; PMID: 9241424
- Mor A, Suliman S, Ben-Yishay R, Yunger S, Brody Y, Shav-Tal Y. Dynamics of single mRNP nucleocytoplasmic transport and export through the nuclear pore in living cells. Nat Cell Biol 2010; 12:543 - 52; http://dx.doi.org/10.1038/ncb2056; PMID: 20453848
- Shav-Tal Y, Gruenbaum Y. Single-molecule dynamics of nuclear mRNA. F1000 Biol Rep 2009; 1:29 - 32; PMID: 20948657
- Siebrasse JP, Veith R, Dobay A, Leonhardt H, Daneholt B, Kubitscheck U. Discontinuous movement of mRNP particles in nucleoplasmic regions devoid of chromatin. Proc Natl Acad Sci U S A 2008; 105:20291 - 6; http://dx.doi.org/10.1073/pnas.0810692105; PMID: 19074261
- Ishihama Y, Funatsu T. Single molecule tracking of quantum dot-labeled mRNAs in a cell nucleus. Biochem Biophys Res Commun 2009; 381:33 - 8; http://dx.doi.org/10.1016/j.bbrc.2009.02.001; PMID: 19351590
- Thompson MA, Casolari JM, Badieirostami M, Brown PO, Moerner WE. Three-dimensional tracking of single mRNA particles in Saccharomyces cerevisiae using a double-helix point spread function. Proc Natl Acad Sci U S A 2010; 107:17864 - 71; http://dx.doi.org/10.1073/pnas.1012868107; PMID: 20921361
- Grünwald D, Martin RM, Buschmann V, Bazett-Jones DP, Leonhardt H, Kubitscheck U, et al. Probing intranuclear environments at the single-molecule level. Biophys J 2008; 94:2847 - 58; http://dx.doi.org/10.1529/biophysj.107.115014; PMID: 18065482
- Bancaud A, Huet S, Daigle N, Mozziconacci J, Beaudouin J, Ellenberg J. Molecular crowding affects diffusion and binding of nuclear proteins in heterochromatin and reveals the fractal organization of chromatin. EMBO J 2009; 28:3785 - 98; http://dx.doi.org/10.1038/emboj.2009.340; PMID: 19927119
- Matsumoto K, Tanaka KJ, Aoki K, Sameshima M, Tsujimoto M. Visualization of the reconstituted FRGY2-mRNA complexes by electron microscopy. Biochem Biophys Res Commun 2003; 306:53 - 8; http://dx.doi.org/10.1016/S0006-291X(03)00909-4; PMID: 12788065
- Batisse J, Batisse C, Budd A, Böttcher B, Hurt E. Purification of nuclear poly(A)-binding protein Nab2 reveals association with the yeast transcriptome and a messenger ribonucleoprotein core structure. J Biol Chem 2009; 284:34911 - 7; http://dx.doi.org/10.1074/jbc.M109.062034; PMID: 19840948
- Skabkin MA, Kiselyova OI, Chernov KG, Sorokin AV, Dubrovin EV, Yaminsky IV, et al. Structural organization of mRNA complexes with major core mRNP protein YB-1. Nucleic Acids Res 2004; 32:5621 - 35; http://dx.doi.org/10.1093/nar/gkh889; PMID: 15494450
- Braga J, Rino J, Carmo-Fonseca M. Photobleaching microscopy reveals the dynamics of mRNA-binding proteins inside live cell nuclei. Prog Mol Subcell Biol 2004; 35:119 - 34; http://dx.doi.org/10.1007/978-3-540-74266-1_6; PMID: 15113082
- Roussel MR, Tang T. Simulation of mRNA diffusion in the nuclear environment. IET Syst Biol 2012; 6:125 - 33; http://dx.doi.org/10.1049/iet-syb.2011.0032; PMID: 23039693
- Boulon S, Basyuk E, Blanchard JM, Bertrand E, Verheggen C. Intra-nuclear RNA trafficking: insights from live cell imaging. Biochimie 2002; 84:805 - 13; http://dx.doi.org/10.1016/S0300-9084(02)01438-4; PMID: 12457567
- Kues T, Dickmanns A, Lührmann R, Peters R, Kubitscheck U. High intranuclear mobility and dynamic clustering of the splicing factor U1 snRNP observed by single particle tracking. Proc Natl Acad Sci U S A 2001; 98:12021 - 6; http://dx.doi.org/10.1073/pnas.211250098; PMID: 11593012
- Grünwald D, Spottke B, Buschmann V, Kubitscheck U. Intranuclear binding kinetics and mobility of single native U1 snRNP particles in living cells. Mol Biol Cell 2006; 17:5017 - 27; http://dx.doi.org/10.1091/mbc.E06-06-0559; PMID: 16987963
- Handwerger KE, Murphy C, Gall JG. Steady-state dynamics of Cajal body components in the Xenopus germinal vesicle. J Cell Biol 2003; 160:495 - 504; http://dx.doi.org/10.1083/jcb.200212024; PMID: 12591912
- Ali GS, Prasad KV, Hanumappa M, Reddy AS. Analyses of in vivo interaction and mobility of two spliceosomal proteins using FRAP and BiFC. PLoS One 2008; 3:e1953; http://dx.doi.org/10.1371/journal.pone.0001953; PMID: 18414657
- Rino J, Carvalho T, Braga J, Desterro JM, Lührmann R, Carmo-Fonseca M. A stochastic view of spliceosome assembly and recycling in the nucleus. PLoS Comput Biol 2007; 3:2019 - 31; http://dx.doi.org/10.1371/journal.pcbi.0030201; PMID: 17967051
- Sapra AK, Ankö ML, Grishina I, Lorenz M, Pabis M, Poser I, et al. SR protein family members display diverse activities in the formation of nascent and mature mRNPs in vivo. Mol Cell 2009; 34:179 - 90; http://dx.doi.org/10.1016/j.molcel.2009.02.031; PMID: 19394295
- Huranová M, Ivani I, Benda A, Poser I, Brody Y, Hof M, et al. The differential interaction of snRNPs with pre-mRNA reveals splicing kinetics in living cells. J Cell Biol 2010; 191:75 - 86; http://dx.doi.org/10.1083/jcb.201004030; PMID: 20921136
- Görnemann J, Kotovic KM, Hujer K, Neugebauer KM. Cotranscriptional spliceosome assembly occurs in a stepwise fashion and requires the cap binding complex. Mol Cell 2005; 19:53 - 63; http://dx.doi.org/10.1016/j.molcel.2005.05.007; PMID: 15989964
- Beyer AL, Osheim YN. Splice site selection, rate of splicing, and alternative splicing on nascent transcripts. Genes Dev 1988; 2:754 - 65; http://dx.doi.org/10.1101/gad.2.6.754; PMID: 3138163
- Wetterberg I, Baurén G, Wieslander L. The intranuclear site of excision of each intron in Balbiani ring 3 pre-mRNA is influenced by the time remaining to transcription termination and different excision efficiencies for the various introns. RNA 1996; 2:641 - 51; PMID: 8756407
- Audibert A, Weil D, Dautry F. In vivo kinetics of mRNA splicing and transport in mammalian cells. Mol Cell Biol 2002; 22:6706 - 18; http://dx.doi.org/10.1128/MCB.22.19.6706-6718.2002; PMID: 12215528
- Singh J, Padgett RA. Rates of in situ transcription and splicing in large human genes. Nat Struct Mol Biol 2009; 16:1128 - 33; http://dx.doi.org/10.1038/nsmb.1666; PMID: 19820712
- Clelland AK, Bales AB, Sleeman JE. Changes in intranuclear mobility of mature snRNPs provide a mechanism for splicing defects in spinal muscular atrophy. J Cell Sci 2012; 125:2626 - 37; http://dx.doi.org/10.1242/jcs.096867; PMID: 22393244
- Gallardo F, Laterreur N, Cusanelli E, Ouenzar F, Querido E, Wellinger RJ, et al. Live cell imaging of telomerase RNA dynamics reveals cell cycle-dependent clustering of telomerase at elongating telomeres. Mol Cell 2011; 44:819 - 27; http://dx.doi.org/10.1016/j.molcel.2011.09.020; PMID: 22152484
- Holt I, Mittal S, Furling D, Butler-Browne GS, Brook JD, Morris GE. Defective mRNA in myotonic dystrophy accumulates at the periphery of nuclear splicing speckles. Genes Cells 2007; 12:1035 - 48; http://dx.doi.org/10.1111/j.1365-2443.2007.01112.x; PMID: 17825047
- Smith KP, Byron M, Johnson C, Xing Y, Lawrence JB. Defining early steps in mRNA transport: mutant mRNA in myotonic dystrophy type I is blocked at entry into SC-35 domains. J Cell Biol 2007; 178:951 - 64; http://dx.doi.org/10.1083/jcb.200706048; PMID: 17846170
- Querido E, Gallardo F, Beaudoin M, Ménard C, Chartrand P. Stochastic and reversible aggregation of mRNA with expanded CUG-triplet repeats. J Cell Sci 2011; 124:1703 - 14; http://dx.doi.org/10.1242/jcs.073270; PMID: 21511730
- Spann P, Feinerman M, Sperling J, Sperling R. Isolation and visualization of large compact ribonucleoprotein particles of specific nuclear RNAs. Proc Natl Acad Sci U S A 1989; 86:466 - 70; http://dx.doi.org/10.1073/pnas.86.2.466; PMID: 2521390
- Iborra FJ, Jackson DA, Cook PR. The path of transcripts from extra-nucleolar synthetic sites to nuclear pores: transcripts in transit are concentrated in discrete structures containing SR proteins. J Cell Sci 1998; 111:2269 - 82; PMID: 9664048
- Panté N, Jarmolowski A, Izaurralde E, Sauder U, Baschong W, Mattaj IW. Visualizing nuclear export of different classes of RNA by electron microscopy. RNA 1997; 3:498 - 513; PMID: 9149231
- Huang S, Deerinck TJ, Ellisman MH, Spector DL. In vivo analysis of the stability and transport of nuclear poly(A)+ RNA. J Cell Biol 1994; 126:877 - 99; http://dx.doi.org/10.1083/jcb.126.4.877; PMID: 7519622
- Dworetzky SI, Feldherr CM. Translocation of RNA-coated gold particles through the nuclear pores of oocytes. J Cell Biol 1988; 106:575 - 84; http://dx.doi.org/10.1083/jcb.106.3.575; PMID: 2450095
- Iborra FJ, Jackson DA, Cook PR. The path of RNA through nuclear pores: apparent entry from the sides into specialized pores. J Cell Sci 2000; 113:291 - 302; PMID: 10633080
- Yang W, Gelles J, Musser SM. Imaging of single-molecule translocation through nuclear pore complexes. Proc Natl Acad Sci U S A 2004; 101:12887 - 92; http://dx.doi.org/10.1073/pnas.0403675101; PMID: 15306682
- Ribbeck K, Görlich D. Kinetic analysis of translocation through nuclear pore complexes. EMBO J 2001; 20:1320 - 30; http://dx.doi.org/10.1093/emboj/20.6.1320; PMID: 11250898
- Yamada J, Phillips JL, Patel S, Goldfien G, Calestagne-Morelli A, Huang H, et al. A bimodal distribution of two distinct categories of intrinsically disordered structures with separate functions in FG nucleoporins. Mol Cell Proteomics 2010; 9:2205 - 24; http://dx.doi.org/10.1074/mcp.M000035-MCP201; PMID: 20368288
- Mehlin H, Daneholt B, Skoglund U. Structural interaction between the nuclear pore complex and a specific translocating RNP particle. J Cell Biol 1995; 129:1205 - 16; http://dx.doi.org/10.1083/jcb.129.5.1205; PMID: 7775568
- Kiseleva E, Goldberg MW, Allen TD, Akey CW. Active nuclear pore complexes in Chironomus: visualization of transporter configurations related to mRNP export. J Cell Sci 1998; 111:223 - 36; PMID: 9405308
- Fiserova J, Richards SA, Wente SR, Goldberg MW. Facilitated transport and diffusion take distinct spatial routes through the nuclear pore complex. J Cell Sci 2010; 123:2773 - 80; http://dx.doi.org/10.1242/jcs.070730; PMID: 20647373
- Mor A, Shav-Tal Y. Dynamics and kinetics of nucleo-cytoplasmic mRNA export. Wiley Interdiscip Rev RNA 2010; 1:388 - 401; http://dx.doi.org/10.1002/wrna.41; PMID: 21956938
- Grünwald D, Singer RH, Rout M. Nuclear export dynamics of RNA-protein complexes. Nature 2011; 475:333 - 41; http://dx.doi.org/10.1038/nature10318; PMID: 21776079
- Stevens BJ, Swift H. RNA transport from nucleus to cytoplasm in Chironomus salivary glands. J Cell Biol 1966; 31:55 - 77; http://dx.doi.org/10.1083/jcb.31.1.55; PMID: 5971975
- Mehlin H, Daneholt B, Skoglund U. Translocation of a specific premessenger ribonucleoprotein particle through the nuclear pore studied with electron microscope tomography. Cell 1992; 69:605 - 13; http://dx.doi.org/10.1016/0092-8674(92)90224-Z; PMID: 1586943
- Skoglund U, Andersson K, Björkroth B, Lamb MM, Daneholt B. Visualization of the formation and transport of a specific hnRNP particle. Cell 1983; 34:847 - 55; http://dx.doi.org/10.1016/0092-8674(83)90542-1; PMID: 6556087
- Kiseleva E, Goldberg MW, Daneholt B, Allen TD. RNP export is mediated by structural reorganization of the nuclear pore basket. J Mol Biol 1996; 260:304 - 11; http://dx.doi.org/10.1006/jmbi.1996.0401; PMID: 8757794
- Siebrasse JP, Kaminski T, Kubitscheck U. Nuclear export of single native mRNA molecules observed by light sheet fluorescence microscopy. Proc Natl Acad Sci U S A 2012; 109:9426 - 31; http://dx.doi.org/10.1073/pnas.1201781109; PMID: 22615357
- Noble KN, Tran EJ, Alcázar-Román AR, Hodge CA, Cole CN, Wente SR. The Dbp5 cycle at the nuclear pore complex during mRNA export II: nucleotide cycling and mRNP remodeling by Dbp5 are controlled by Nup159 and Gle1. Genes Dev 2011; 25:1065 - 77; http://dx.doi.org/10.1101/gad.2040611; PMID: 21576266
- Verschure PJ, van der Kraan I, Manders EM, Hoogstraten D, Houtsmuller AB, van Driel R. Condensed chromatin domains in the mammalian nucleus are accessible to large macromolecules. EMBO Rep 2003; 4:861 - 6; http://dx.doi.org/10.1038/sj.embor.embor922; PMID: 12947417
- Seksek O, Biwersi J, Verkman AS. Translational diffusion of macromolecule-sized solutes in cytoplasm and nucleus. J Cell Biol 1997; 138:131 - 42; http://dx.doi.org/10.1083/jcb.138.1.131; PMID: 9214387
- Cremer T, Cremer C. Chromosome territories, nuclear architecture and gene regulation in mammalian cells. Nat Rev Genet 2001; 2:292 - 301; http://dx.doi.org/10.1038/35066075; PMID: 11283701
- Zachar Z, Kramer J, Mims IP, Bingham PM. Evidence for channeled diffusion of pre-mRNAs during nuclear RNA transport in metazoans. J Cell Biol 1993; 121:729 - 42; http://dx.doi.org/10.1083/jcb.121.4.729; PMID: 8491768
- Fakan S. Perichromatin fibrils are in situ forms of nascent transcripts. Trends Cell Biol 1994; 4:86 - 90; http://dx.doi.org/10.1016/0962-8924(94)90180-5; PMID: 14731598
- Spector DL. The dynamics of chromosome organization and gene regulation. Annu Rev Biochem 2003; 72:573 - 608; http://dx.doi.org/10.1146/annurev.biochem.72.121801.161724; PMID: 14527325
- Niedojadlo J, Perret-Vivancos C, Kalland KH, Cmarko D, Cremer T, van Driel R, et al. Transcribed DNA is preferentially located in the perichromatin region of mammalian cell nuclei. Exp Cell Res 2011; 317:433 - 44; http://dx.doi.org/10.1016/j.yexcr.2010.10.026; PMID: 21056558
- Cook PR. A model for all genomes: the role of transcription factories. J Mol Biol 2010; 395:1 - 10; http://dx.doi.org/10.1016/j.jmb.2009.10.031; PMID: 19852969
- Carter KC, Bowman D, Carrington W, Fogarty K, McNeil JA, Fay FS, et al. A three-dimensional view of precursor messenger RNA metabolism within the mammalian nucleus. Science 1993; 259:1330 - 5; http://dx.doi.org/10.1126/science.8446902; PMID: 8446902
- Bridger JM, Kalla C, Wodrich H, Weitz S, King JA, Khazaie K, et al. Nuclear RNAs confined to a reticular compartment between chromosome territories. Exp Cell Res 2005; 302:180 - 93; http://dx.doi.org/10.1016/j.yexcr.2004.07.038; PMID: 15561100
- Reichenzeller M, Burzlaff A, Lichter P, Herrmann H. In vivo observation of a nuclear channel-like system: evidence for a distinct interchromosomal domain compartment in interphase cells. J Struct Biol 2000; 129:175 - 85; http://dx.doi.org/10.1006/jsbi.2000.4224; PMID: 10806067
- Zirbel RM, Mathieu UR, Kurz A, Cremer T, Lichter P. Evidence for a nuclear compartment of transcription and splicing located at chromosome domain boundaries. Chromosome Res 1993; 1:93 - 106; http://dx.doi.org/10.1007/BF00710032; PMID: 8143096
- Lawrence JB, Singer RH, Marselle LM. Highly localized tracks of specific transcripts within interphase nuclei visualized by in situ hybridization. Cell 1989; 57:493 - 502; http://dx.doi.org/10.1016/0092-8674(89)90924-0; PMID: 2541917
- Xing YG, Lawrence JB. Preservation of specific RNA distribution within the chromatin-depleted nuclear substructure demonstrated by in situ hybridization coupled with biochemical fractionation. J Cell Biol 1991; 112:1055 - 63; http://dx.doi.org/10.1083/jcb.112.6.1055; PMID: 1705562
- Xing Y, Johnson CV, Dobner PR, Lawrence JB. Higher level organization of individual gene transcription and RNA splicing. Science 1993; 259:1326 - 30; http://dx.doi.org/10.1126/science.8446901; PMID: 8446901
- Dirks RW, Daniël KC, Raap AK. RNAs radiate from gene to cytoplasm as revealed by fluorescence in situ hybridization. J Cell Sci 1995; 108:2565 - 72; PMID: 7593297
- Krull S, Dörries J, Boysen B, Reidenbach S, Magnius L, Norder H, et al. Protein Tpr is required for establishing nuclear pore-associated zones of heterochromatin exclusion. EMBO J 2010; 29:1659 - 73; http://dx.doi.org/10.1038/emboj.2010.54; PMID: 20407419
- Albiez H, Cremer M, Tiberi C, Vecchio L, Schermelleh L, Dittrich S, et al. Chromatin domains and the interchromatin compartment form structurally defined and functionally interacting nuclear networks. Chromosome Res 2006; 14:707 - 33; http://dx.doi.org/10.1007/s10577-006-1086-x; PMID: 17115328
- Tutucci E, Stutz F. Keeping mRNPs in check during assembly and nuclear export. Nat Rev Mol Cell Biol 2011; 12:377 - 84; http://dx.doi.org/10.1038/nrm3119; PMID: 21602906
- Zhou Z, Luo MJ, Straesser K, Katahira J, Hurt E, Reed R. The protein Aly links pre-messenger-RNA splicing to nuclear export in metazoans. Nature 2000; 407:401 - 5; http://dx.doi.org/10.1038/35030160; PMID: 11014198
- Strässer K, Masuda S, Mason P, Pfannstiel J, Oppizzi M, Rodriguez-Navarro S, et al. TREX is a conserved complex coupling transcription with messenger RNA export. Nature 2002; 417:304 - 8; http://dx.doi.org/10.1038/nature746; PMID: 11979277
- Stutz F, Bachi A, Doerks T, Braun IC, Séraphin B, Wilm M, et al. REF, an evolutionary conserved family of hnRNP-like proteins, interacts with TAP/Mex67p and participates in mRNA nuclear export. RNA 2000; 6:638 - 50; http://dx.doi.org/10.1017/S1355838200000078; PMID: 10786854
- Bachi A, Braun IC, Rodrigues JP, Panté N, Ribbeck K, von Kobbe C, et al. The C-terminal domain of TAP interacts with the nuclear pore complex and promotes export of specific CTE-bearing RNA substrates. RNA 2000; 6:136 - 58; http://dx.doi.org/10.1017/S1355838200991994; PMID: 10668806
- Katahira J, Strässer K, Podtelejnikov A, Mann M, Jung JU, Hurt E. The Mex67p-mediated nuclear mRNA export pathway is conserved from yeast to human. EMBO J 1999; 18:2593 - 609; http://dx.doi.org/10.1093/emboj/18.9.2593; PMID: 10228171
- Wickramasinghe VO, McMurtrie PI, Mills AD, Takei Y, Penrhyn-Lowe S, Amagase Y, et al. mRNA export from mammalian cell nuclei is dependent on GANP. Curr Biol 2010; 20:25 - 31; http://dx.doi.org/10.1016/j.cub.2009.10.078; PMID: 20005110
- Le Hir H, Izaurralde E, Maquat LE, Moore MJ. The spliceosome deposits multiple proteins 20-24 nucleotides upstream of mRNA exon-exon junctions. EMBO J 2000; 19:6860 - 9; http://dx.doi.org/10.1093/emboj/19.24.6860; PMID: 11118221
- Le Hir H, Gatfield D, Izaurralde E, Moore MJ. The exon-exon junction complex provides a binding platform for factors involved in mRNA export and nonsense-mediated mRNA decay. EMBO J 2001; 20:4987 - 97; http://dx.doi.org/10.1093/emboj/20.17.4987; PMID: 11532962
- Björk P, Jin S, Zhao J, Singh OP, Persson JO, Hellman U, et al. Specific combinations of SR proteins associate with single pre-messenger RNAs in vivo and contribute different functions. J Cell Biol 2009; 184:555 - 68; http://dx.doi.org/10.1083/jcb.200806156; PMID: 19221196
- Schmidt U, Richter K, Berger AB, Lichter P. In vivo BiFC analysis of Y14 and NXF1 mRNA export complexes: preferential localization within and around SC35 domains. J Cell Biol 2006; 172:373 - 81; http://dx.doi.org/10.1083/jcb.200503061; PMID: 16431928
- Cullen BR. Nuclear RNA export. J Cell Sci 2003; 116:587 - 97; http://dx.doi.org/10.1242/jcs.00268; PMID: 12538759
- Lang I, Scholz M, Peters R. Molecular mobility and nucleocytoplasmic flux in hepatoma cells. J Cell Biol 1986; 102:1183 - 90; http://dx.doi.org/10.1083/jcb.102.4.1183; PMID: 2420804
- Braga J, Desterro JM, Carmo-Fonseca M. Intracellular macromolecular mobility measured by fluorescence recovery after photobleaching with confocal laser scanning microscopes. Mol Biol Cell 2004; 15:4749 - 60; http://dx.doi.org/10.1091/mbc.E04-06-0496; PMID: 15292455
- English BP, Hauryliuk V, Sanamrad A, Tankov S, Dekker NH, Elf J. Single-molecule investigations of the stringent response machinery in living bacterial cells. Proc Natl Acad Sci U S A 2011; 108:E365 - 73; http://dx.doi.org/10.1073/pnas.1102255108; PMID: 21730169
- Golding I, Cox EC. Physical nature of bacterial cytoplasm. Phys Rev Lett 2006; 96:098102; http://dx.doi.org/10.1103/PhysRevLett.96.098102; PMID: 16606319
- Schnell S, Hancock R. The intranuclear environment. Methods Mol Biol 2008; 463:3 - 19; http://dx.doi.org/10.1007/978-1-59745-406-3_1; PMID: 18951157
- Richter K, Nessling M, Lichter P. Experimental evidence for the influence of molecular crowding on nuclear architecture. J Cell Sci 2007; 120:1673 - 80; http://dx.doi.org/10.1242/jcs.03440; PMID: 17430977
- Hameed FM, Rao M, Shivashankar GV. Dynamics of passive and active particles in the cell nucleus. PLoS One 2012; 7:e45843; http://dx.doi.org/10.1371/journal.pone.0045843; PMID: 23077497
- Dross N, Spriet C, Zwerger M, Müller G, Waldeck W, Langowski J. Mapping eGFP oligomer mobility in living cell nuclei. PLoS One 2009; 4:e5041; http://dx.doi.org/10.1371/journal.pone.0005041; PMID: 19347038
- Hinde E, Cardarelli F, Digman MA, Gratton E. In vivo pair correlation analysis of EGFP intranuclear diffusion reveals DNA-dependent molecular flow. Proc Natl Acad Sci U S A 2010; 107:16560 - 5; http://dx.doi.org/10.1073/pnas.1006731107; PMID: 20823232
- Speese SD, Ashley J, Jokhi V, Nunnari J, Barria R, Li Y, et al. Nuclear envelope budding enables large ribonucleoprotein particle export during synaptic Wnt signaling. Cell 2012; 149:832 - 46; http://dx.doi.org/10.1016/j.cell.2012.03.032; PMID: 22579286
- Montpetit B, Weis K. Cell biology. An alternative route for nuclear mRNP export by membrane budding. Science 2012; 336:809 - 10; http://dx.doi.org/10.1126/science.1222243; PMID: 22605737
- Muranyi W, Haas J, Wagner M, Krohne G, Koszinowski UH. Cytomegalovirus recruitment of cellular kinases to dissolve the nuclear lamina. Science 2002; 297:854 - 7; http://dx.doi.org/10.1126/science.1071506; PMID: 12161659
- Stewart M. Ratcheting mRNA out of the nucleus. Mol Cell 2007; 25:327 - 30; http://dx.doi.org/10.1016/j.molcel.2007.01.016; PMID: 17289581
- Nachury MV, Weis K. The direction of transport through the nuclear pore can be inverted. Proc Natl Acad Sci U S A 1999; 96:9622 - 7; http://dx.doi.org/10.1073/pnas.96.17.9622; PMID: 10449743
- Kopito RB, Elbaum M. Reversibility in nucleocytoplasmic transport. Proc Natl Acad Sci U S A 2007; 104:12743 - 8; http://dx.doi.org/10.1073/pnas.0702690104; PMID: 17646647
- Booth-Gauthier EA, Alcoser TA, Yang G, Dahl KN. Force-induced changes in subnuclear movement and rheology. Biophys J 2012; 103:2423 - 31; http://dx.doi.org/10.1016/j.bpj.2012.10.039; PMID: 23260044
- Dahl KN, Kalinowski A. Nucleoskeleton mechanics at a glance. J Cell Sci 2011; 124:675 - 8; http://dx.doi.org/10.1242/jcs.069096; PMID: 21321324
- Chen BP, Li YS, Zhao Y, Chen KD, Li S, Lao J, et al. DNA microarray analysis of gene expression in endothelial cells in response to 24-h shear stress. Physiol Genomics 2001; 7:55 - 63; http://dx.doi.org/10.1006/geno.2001.6511; PMID: 11595792
- Poh YC, Shevtsov SP, Chowdhury F, Wu DC, Na S, Dundr M, et al. Dynamic force-induced direct dissociation of protein complexes in a nuclear body in living cells. Nat Commun 2012; 3:866; http://dx.doi.org/10.1038/ncomms1873; PMID: 22643893
- Tripathi V, Ellis JD, Shen Z, Song DY, Pan Q, Watt AT, et al. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol Cell 2010; 39:925 - 38; http://dx.doi.org/10.1016/j.molcel.2010.08.011; PMID: 20797886
- Wilusz JE, Freier SM, Spector DL. 3′ end processing of a long nuclear-retained noncoding RNA yields a tRNA-like cytoplasmic RNA. Cell 2008; 135:919 - 32; http://dx.doi.org/10.1016/j.cell.2008.10.012; PMID: 19041754
- Audas TE, Jacob MD, Lee S. Immobilization of proteins in the nucleolus by ribosomal intergenic spacer noncoding RNA. Mol Cell 2012; 45:147 - 57; http://dx.doi.org/10.1016/j.molcel.2011.12.012; PMID: 22284675
- Borah S, Darricarrère N, Darnell A, Myoung J, Steitz JA. A viral nuclear noncoding RNA binds re-localized poly(A) binding protein and is required for late KSHV gene expression. PLoS Pathog 2011; 7:e1002300; http://dx.doi.org/10.1371/journal.ppat.1002300; PMID: 22022268
- Borah S, Nichols LA, Hassman LM, Kedes DH, Steitz JA. Tracking expression and subcellular localization of RNA and protein species using high-throughput single cell imaging flow cytometry. RNA 2012; 18:1573 - 9; http://dx.doi.org/10.1261/rna.033126.112; PMID: 22745225
- Lim F, Downey TP, Peabody DS. Translational repression and specific RNA binding by the coat protein of the Pseudomonas phage PP7. J Biol Chem 2001; 276:22507 - 13; http://dx.doi.org/10.1074/jbc.M102411200; PMID: 11306589
- Schönberger J, Hammes UZ, Dresselhaus T. In vivo visualization of RNA in plants cells using the λN₂₂ system and a GATEWAY-compatible vector series for candidate RNAs. Plant J 2012; 71:173 - 81; http://dx.doi.org/10.1111/j.1365-313X.2012.04923.x; PMID: 22268772
- Hocine S, Raymond P, Zenklusen D, Chao JA, Singer RH. Single-molecule analysis of gene expression using two-color RNA labeling in live yeast. Nat Methods 2013; 10:119 - 21; http://dx.doi.org/10.1038/nmeth.2305; PMID: 23263691
- Larson DR, Zenklusen D, Wu B, Chao JA, Singer RH. Real-time observation of transcription initiation and elongation on an endogenous yeast gene. Science 2011; 332:475 - 8; http://dx.doi.org/10.1126/science.1202142; PMID: 21512033
- Lange S, Katayama Y, Schmid M, Burkacky O, Bräuchle C, Lamb DC, et al. Simultaneous transport of different localized mRNA species revealed by live-cell imaging. Traffic 2008; 9:1256 - 67; http://dx.doi.org/10.1111/j.1600-0854.2008.00763.x; PMID: 18485054
- Daigle N, Ellenberg J. LambdaN-GFP: an RNA reporter system for live-cell imaging. Nat Methods 2007; 4:633 - 6; http://dx.doi.org/10.1038/nmeth1065; PMID: 17603490
- Wu B, Chao JA, Singer RH. Fluorescence fluctuation spectroscopy enables quantitative imaging of single mRNAs in living cells. Biophys J 2012; 102:2936 - 44; http://dx.doi.org/10.1016/j.bpj.2012.05.017; PMID: 22735544