Abstract
The nuclear pore complex (NPC), which provides the permeable and selective transport path between the nucleus and cytoplasm of eukaryotic cells, allows both the passive diffusion of small molecules in a signal-independent manner and the transport receptor-facilitated translocation of cargo molecules in a signal-dependent manner. However, the spatial and functional relationships between these two transport pathways, which represent critical information for unraveling the fundamental nucleocytoplasmic transport mechanism, remain in dispute. The direct experimental examination of passive and facilitated transport with a high spatiotemporal resolution under real-time trafficking conditions in native NPCs is still difficult. To address this issue and further define these transport mechanisms, we recently developed single-point edge-excitation sub-diffraction (SPEED) microscopy and a deconvolution algorithm to directly map both passive and facilitated transport routes in three dimensions (3D) in native NPCs. Our findings revealed that passive and facilitated transport occur through spatially distinct transport routes. Signal-independent small molecules exhibit a high probability of passively diffusing through an axial central viscous channel, while transport receptors and their cargo complexes preferentially travel through the periphery, around this central channel, after interacting with phenylalanine-glycine (FG) filaments. Strikingly, these two distinct transport zones are not completely separate either spatially or functionally. Instead, their conformations are closely correlated and simultaneously regulated. In this review, we will specifically highlight a detailed procedure for 3D mapping of passive and facilitated transport routes, demonstrate the correlation between these two distinct pathways, and finally, speculate regarding the regulation of the transport pathways driven by the conformational changes of FG filaments in NPCs.
NPC Structure and Function
In eukaryotic cells, the double lipid bilayer of the nuclear envelope (NE) separates the nucleus from the cytoplasm.Citation1,Citation2 Transcription in the nucleus and translation in the cytoplasm require eukaryotic cells to perform highly regulated, massive transport between the two segregated compartments.Citation3-Citation5 Although large ribonucleoprotein complexes were recently reported to be exported from the nucleus through nuclear membrane budding,Citation6 the majority of the bidirectional trafficking of macromolecules between the nucleus and the cytoplasm is still believed to be mediated by thousands of nuclear pore complexes (NPCs) embedded in the NE.Citation7-Citation16 As revealed by electron microscopy, the architecture of the NPC consists of a central scaffold region with an approximate length of 40–90 nm and an inner diameter of 50 nm, multiple flexible fibrils extending approximately 50 nm into the cytoplasm and a basket structure that protrudes approximately 75 nm into the nucleus.Citation17-Citation21 The NPC is a large assemblage composed of approximately 30 different proteins, each of which is present in multiples of eight copies, known as nucleoporins (Nups). Approximately one-third of these Nups possess a native unfolded structure with domains that are rich in phenylalanine-glycine (FG) repeats. These FG Nups form a selective permeability barrier in the NPC that allows two modes of transport to occur: the passive diffusion of small molecules (< 40 kDa) in a signal-independent manner and the facilitated translocation of cargo molecules (up to ~25–50 MDa) in a signal-dependent manner.Citation22-Citation26 The passive diffusion of small molecules through the FG barrier in the NPC suggests that the available spatial channels (or gaps) in the barrier are sufficiently large for small molecules to diffuse through them, without consuming any chemical energy. In contrast, in the facilitated translocation pathway for signal-dependent cargo molecules, these molecules either permeate the NPC with a low efficiency or are repelled from the NPC, unless transport receptors mediate their traversal through the NPC via interacting with FG Nups.Citation27-Citation29 The initial association and final dissociation of the in-transit cargo complexes are normally guided by a RanGTP (or RanGDP) concentration gradient across the NE.Citation30,Citation31 Although a great deal of knowledge has been obtained about nucleocytoplasmic transport, the fundamental transport mechanism underlying the interaction of in-transit molecules with the selective barrier that allows them to complete their journey through the NPC remains elusive.
Spatial Transport Routes through the NPC
To maintain cellular viability and growth, the NPCs must mediate massive transport of various molecules between the cytoplasm and the nucleus. The sizes of in-transit molecules can be as small as half a nanometer or as large as several tens of nanometers.Citation32-Citation35 It has been estimated that a single NPC could mediate the transport of up to 20 MDa of materials between the cytoplasm and the nucleus per second.Citation4 Given the physical confinement of the nuclear pores and the millimolar FG filaments that fill them,Citation36 in-transit molecules must traverse a very crowded filamentous environment, especially in the central scaffold region of the NPC, which has an inner diameter of approximately 50 nm. How passive and facilitated transport share these compact filamentous pores and whether they affect each other spatially and functionally remain a matter of dispute in terms of both experimental observations and proposed transport models.Citation24,Citation37-Citation42
Many approaches have been applied to determine the transport routes present in NPCs. Originally, passive and facilitated transport were suggested to be separated based on three-dimensional models constructed using transmission electron microscopy (TEM) data.Citation43-Citation45 Later, similar studies further indicated that these two transport modes were functionally independent, without any competition occurring between them.Citation46,Citation47 However, at the same time, many other researchers argued against the above conclusions because they observed common pathways shared by passive and facilitated transport, not only spatially but also in terms of function.Citation48-Citation51
The above disputes are reflected in the proposed transport models. Six currently prevailing transport models in this field have been listed in . The disputes between these models focus on at least three critical questions: (1) is there a single diffusion channel or are there multiple channels for small molecules in the NPC, (2) do passive and facilitated transport display spatially and functionally separate or shared pathways and (3) do the transport receptors collapse or dissolve the selective barrier formed by FG filaments in the NPC? The oily-spaghetti and reduction of dimensionality (ROD) models predict that an axial central channel, approximately 10–15 nm in diameter, exists in the NPC, allowing the passive diffusion of small molecules.Citation37,Citation38 These models, together with the virtual gating and the polymer brush models,Citation24,Citation39 predict that the binding of transport receptors to FG repeats promotes efficient facilitated translocation of macromolecules due to overcoming the entropic barrier of the NPC or collapsing FG filaments toward their anchors or through two-dimensional (2D) movements on the FG repeat-coated inner wall of the NPC. In contrast, the selective phase/hydrogel model proposes that weak hydrophobic interactions between FG repeats create a sieve-like hydrogel meshwork within the NPC and that small molecules passively diffuse through numerous randomly distributed small holes (≤ 2.6 nm in diameter) in this mesh.Citation40,Citation41 In this model, transport receptors and their cargo complexes achieve efficient translocation through the NPC by dissolving in the FG hydrogel network, rather than by collapsing the FG filaments toward their anchors. Finally, the forest model posits that two separate transport zones could form in the NPC for passive diffusion and facilitated transport, depending on interactions between FG domains.Citation42
Table 1. Although no current model covers all of the known properties of nuclear transport, some models do make different, testable predictions for passive and active transport through the NPC
3D Mapping of Nucleocytoplasmic Transport via SPEED Microscopy
To address the long-lasting disputes described above and to distinguish among the proposed transport models, direct mapping of the routes of passive and facilitated transport in native NPCs is required. However, the size of the NPC, the fast, inherently 3D movements of molecules through the NPC, and the natively unfolded FG Nups that fill the nuclear pore constitute a challenge to current imaging techniques. First, the 200-nm long NPC, which is just below the diffraction limit of light microscopy, requires imaging approaches with a high spatial resolution, such as electron microscopy (EM) or super-resolution light microscopy.Citation52-Citation69 Nevertheless, while the spatial resolution of EM can be as high as several nanometers, the fixed or frozen samples of NPCs used in EM imaging prevent real-time tracking of molecules through the NPC. Alternatively, super-resolution light microscopy is usually capable of capturing dynamic, real-time images at a sub-diffraction-limit spatial resolution of 20–80 nm, but temporal resolution is sacrificed due to adapting minute- or even hour-long detection times, which is far above the millisecond nuclear transport time.Citation54,Citation59,Citation60,Citation63-Citation65 Furthermore, the inherently 3D movements of in-transit molecules in the NPC require an imaging technique that is able to follow the molecules through the NPC in 3D, within their millisecond transport times. Unfortunately, the currently available 3D imaging methods generally cannot capture or distinguish such rapid diffusion in a sub-micrometer bio-cavity such as the NPC.Citation70-Citation75 Finally, the complexity of natively unfolded FG Nups in the NPC makes mimicking real nuclear transport in artificial pores challenging.Citation76-Citation78 Therefore, an imaging technique that can map real-time nuclear transport at an exceptionally high spatiotemporal resolution in 3D in the NPCs of living cells is urgently needed.
SPEED microscopy
To meet the above requirements for the mapping of nuclear transport, an innovative imaging approach that combines single-particle tracking and a deconvolution algorithm has recently been developed in our laboratory.Citation79-Citation82 First, single-particle tracking of in-transit molecules through a single NPC is realized using single-point edge-excitation sub-diffraction (SPEED) microscopy. The first goal of SPEED microscopy is to illuminate a single NPC in the NE in permeabilized and living cells. In SPEED microcopy, a laser beam is shifted from the center of an objective to enhance the inclined diffraction-limited spot illumination volume at the focal plane of the objective at an angle of 45° from the perpendicular direction (). Such an inclined illumination volume generates a point spread function (PSF) smaller than the average distance between the nearest neighboring nuclear pores in three dimensions.Citation82 For example, if a 488-nm laser beam is used to illuminate a single GFP-labeled NPC in cells, the resultant illumination volume associated with a 260-nm PSF in three dimensions is sufficiently small to selectively excite a single NPC from thousands of NPCs on the NE with an average neighboring distance of 400–500 nm.Citation79 Next, a second laser with a different wavelength following the same beam path as the 488-nm laser is used to conduct single-particle tracking of labeled in-transit molecules through this illuminated NPC.Citation79 Noteworthy, the major difference between SPEED microscopy and a previous reported highly inclined and laminated optical sheet microscopy is the different illumination volume formed at the focal plane of objective.Citation90 The former provides a diffraction-limit point excitation and the latter generates a plane illumination.
Figure 1. SPEED microscopy imaging of the nuclear transport of single molecules through single native NPCs at the equator of the NE. (A) Illumination of a single NPC at the equator of a cell via SPEED microscopy. Single in-transit molecules (red dots) traversing a single GFP-labeled NPC (blue) imaged using an inclined illumination point spread function (the iPSF forms an angle of 45° from the z direction) at the equatorial plane of the HeLa cell nucleus in the focal plane (between the double light blue lines). C, cytoplasm; N, nucleus. (B) 2D superimposed spatial locations of 10 kDa dextran molecules passively diffusing through the NPC. Superimposed plots of the spatial locations of single dextran molecules, primarily within a rectangular area of 240x160 nm around the centroid of the NPC. (C) 2D superimposed spatial locations of Imp β1 molecules in the NPC. (D) 2D superimposed spatial locations of Importin α1/Imp β1/NLS-2xGFP complexes in the NPC.
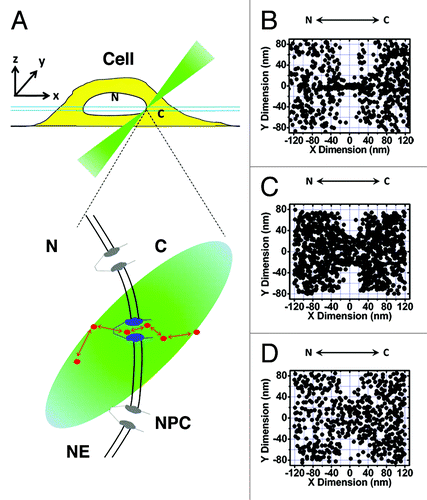
The illumination of a single NPC in the NE provides the following advantages.Citation79-Citation82 (1) Single NPCs in cells can be directly localized with a 1–3 nm spatial localization precision, rather than localizing the partial or entire NE, as in previous measurements.Citation80 (2) The diffraction limit illumination volume of SPEED microscopy enables a very high detection speed with a CCD camera. (3) A better spatial localization precision is generated for single moving molecules due to minimizing the diffusion-driven Gaussian width. As an example, we achieved a localization precision of 9 nm for immobile molecules and of 10 nm for moving molecules at 400 μs by collecting ~1,100 photons from four Alexa fluor 647-labeled Imp β1 molecules.Citation82 (4) Accurate determination of the relative locations of the trajectories of single in-transit molecules and the centroid of the NPC results in determination of the real-time 2D spatial distribution of molecular locations in NPCs. (5) Single-pore imaging provides the essential conditions for further development of a deconvolution algorithm to realize 3D mapping of transport routes in the NPC.
Side view of nuclear transport via SPEED microscopy
To distinguish the import and export events occurring through the NPCs, we employed SPEED microscopy to illuminate single NPCs at the equator of the NE and to track single in-transit molecules through NPCs.Citation79,Citation82 After the centroids of single NPCs and the single-molecule trajectories of in-transit molecules through these NPCs were overlaid, a 2D plot of the spatial locations of in-transit molecules in the NPC was obtained. With knowledge of the starting and ending locations for each transport event, the transport direction (import or export), transport time (dwell time in the NPC) and transport efficiency (percentage of molecules successfully traversing the NPC) can be determined. The 2D spatial locations of in-transit molecules were further converted into 2D probability density maps after filtering using the Gaussian blur function. However, the obtained 2D probability density maps cannot be directly used to distinguish spatial transport routes. As shown in , the 2D maps generated for passive diffusion and facilitated transport revealed that their spatial locations display different distributions but also share some overlapping regions in the NPC. If the differences between the two transport routes are emphasized, a conclusion of distinct routes might be drawn, whereas if the overlap between the two distributions is focused upon, it would be inferred that the two transport pathways share space in the NPC. Therefore, whether passive and facilitated transport display spatially separate or shared transport routes remains unclear in the obtained 2D spatial location maps. Ultimately, 3D images of the two pathways are needed to allow them to be compared both spatially and functionally.
Deconvolution algorithm
In principle, the 3D locations of single molecules in the NPC can be defined using either a Cartesian coordinate system (x, y, z) or a cylinder coordinate system (r, x, θ) (). When imaged with a light microscope, the 3D locations of single molecules are projected onto their 2D locations in the image plane of the microscope (). This projection is actually a process involving convolution of the 3D information. The obtained 2D distribution retains all of the information on 3D locations and can be deconvoluted (or back-projected) to recover the 3D information with either accepted assumptions or additional information obtained from other sources. Moreover, in our experiments, the 2D spatial locations of transport events in multiple NPCs were superimposed to obtain a sufficient number of locations to generate a 2D probability density map. Superposition was conducted using the centroid of each NPC as the reference point.Citation79,Citation82
Figure 2. Procedure for application of the 2D to 3D deconvolution algorithm. (A) 3D locations of single molecules (red dots) in the NPC can be shown using either a Cartesian (x, y, z) or cylinder (r, x, θ) coordinate system. (B) Spatial locations of single molecules superimposed from multiple NPCs shown using a Cartesian coordinate system. After imaging via light microscopy, the 3D spatial locations are projected as 2D distributions in the focal plane of the objective. The 3D (x, y, z) coordinates are simplified into the corresponding 2D (x, y) coordinates. The histogram in the y direction at each δx is obtained as F(y). (C) Spatial locations of single molecules superimposed from multiple NPCs shown using a cylinder coordinate system. The (r, x) coordinates of each spatial location are defined according to the centroid of the NPC when molecular locations are superimposed, although locations in the θ direction are arbitrarily overlaid. In principle, the large number of arbitrarily superimposed locations in the θ direction generates a uniform distribution in the θ direction along the ring defined at one (r, x). Therefore, the 3D (r, x, θ) coordinates are simplified as 2D (r, x) coordinates. The histogram in the r direction at δx is constructed as f(r). The relationship between F(y) and f(r) is defined to conduct the deconvolution calculation.Citation79,Citation82
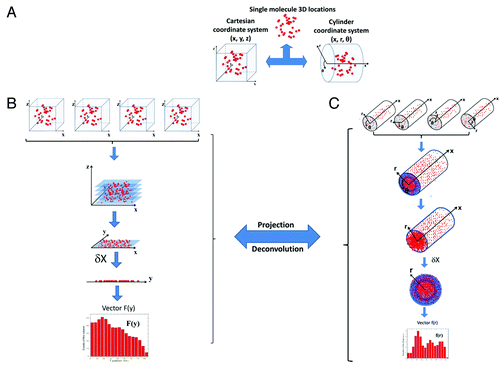
As shown in , under a Cartesian coordinate system, the 3D locations (x, y, z) of single molecules in an NPC are projected as their 2D spatial distribution (x, y) after microscopy imaging. Under a cylinder coordinate system (), the 3D information on spatial locations (r, x, θ) is also simplified based on the superposition of multiple NPCs. Specifically, the centroid of each NPC can be well defined as (r = 0, x = 0), but independent of θ in the cylinder coordinate system. When multiple NPCs are overlaid based on the centroid of each NPC (the orientations of all of these NPCs were precisely defined in our experiments), the relative locations of single-molecule trajectories in (r, x) are reliably localized, but not the θ coordinate. The distribution of single-molecule locations along the θ dimension is then averaged to make it uniform, and thus, θ becomes a constant for each single-molecule location, as (r, x, constant). Therefore, the 3D coordinate (r, x, θ) of the cylinder coordinate system is simplified as the 2D coordinate (r, x).
Finally, a deconvolution algorithm between the 2D Cartesian coordinates (x, y) and the 2D cylinder coordinates (r, x) was developed using 1D histogramized vectors, F(y) and f(r) (). By means of the deconvolution process, the coordinates (r, x) are obtained from the Cartesian coordinates (x, y), and the deconvoluted 3D probability density map of in-transit molecules in the NPC is plotted with 3D modeling software ().Citation79,Citation82 Recently, the distribution of single-molecule locations along the θ dimension in each NPC was experimentally confirmed to be approximately homogenous.Citation79
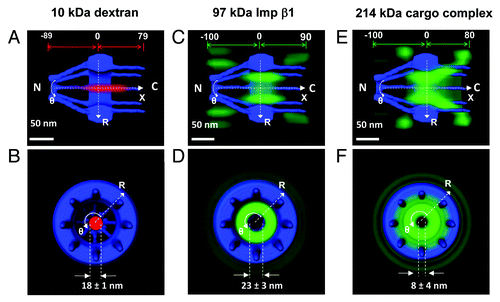
Figure 3. 3D routes of passive and facilitated transport in the NPC. (A–B) By applying a 2D to 3D deconvolution process, a 3D probability density map for 10 kDa dextran (red cloud; a brighter color indicates a higher density) was generated from both a side view and a top view, superimposed on the NPC architecture (blue), in which the distance from and the diameter in the central plane of the NPC was demonstrated. N, the nucleoplasmic side of the NPC. C, the cytoplasmic side of the NPC. (C–D) 3D probability density map of Imp β1 in the NPC. The 3D probability density map of Imp β1 is shown as a green cloud, with a brighter color indicating a higher density. (E–F) 3D probability density map for labeled NLS-2xGFP forming an import complex with Imp α and Imp β1.
3D mapping of spatial transport routes for passive diffusion vs. transport receptor-facilitated translocation in NPCs
Using the above deconvolution process, the 2D spatial distributions of the passive diffusion and transport receptor-facilitated transport pathways were converted into 3D probability density maps ().Citation79,Citation82 The 3D spatial probability density maps revealed the probable spatial dwelling locations for the targeted in-transit molecules in the NPCs, which actually reflected the real-time transport routes of these molecules through the NPCs. Clearly, small molecules exhibit a high probability of passively diffusing through an axial central path (). Conversely, transport receptor-facilitated translocation largely avoids this central channel and preferentially occurs through the periphery around the central path (). Fundamental similarities and obvious differences were observed when comparing the cargo-free and cargo-bound transport receptor Imp β1 (). The efficient nuclear transport of large cargo complexes is realized through interactions between Imp β1 and the FG Nups, and thus, these cargo complexes have to follow the spatial path of Imp β1. Simultaneously, the altered binding affinity of Imp β1 to the FG Nups caused by cargo binding, the large molecular size of cargo complex and the orientation of the cargo complex in the pore could result in the differences in the spatial transport routes for cargo-free and cargo-bound Imp β1.Citation82 Moreover, the existence of distinct spatial transport routes for passive and facilitated transport has been repeatedly confirmed for the transport routes of other small molecules and transport receptors.Citation79 Thus, the 3D imaging approach clearly provided strong evidence supporting a single central channel configuration and the existence of distinct spatial transport routes in the NPC.
Bottom view of nuclear transport via SPEED microscopy
In addition to obtaining a side view of nuclear transport, single NPCs can be illuminated from the bottom (see supporting information in ref. Citation79). The advantage of this approach is that it allows direct observation of the spatial routes of passive and facilitated transport in a cross-section of the NPC. Based on the Gaussian widths of the in-transit molecules that indicate their positions in the axial direction of nucleocytoplasmic transport, whether these transiting molecules are inside or outside the NPC were determined. The single-molecule trajectories of the events inside the NPC are then superimposed to obtain their spatial distribution in a cross-section of the NPC. The spatial distributions of small dextran molecules and Imp β1 clearly indicated their distinct spatial transport pathways in the NPC cross-section. The small molecules were concentrated in the central region, whereas Imp β1 preferentially remained in peripheral regions and seldom occupied the central channel.Citation79 These results agreed very well with the cross-sectional view of passive and facilitated transport routes obtained from the deconvolution process () and supported the existence of a configuration involving distinct spatial transport routes for passive and facilitated transport in the NPC.
Distinct, but not Completely Separate Transport Pathways in the NPC
Distinct spatial routes have been identified for passive and facilitated transport in native NPCs, but whether they are spatially and functionally separated remains a critical question to be answered. First, it appears that there is no rigid line that spatially separates the two transport zones in the NPC, and instead, there is spatial overlap between them at their interface ().Citation79 Furthermore, the overlapping region depends on the size of small passively diffusing molecules, as it was observed that smaller molecules passively permeate deeper into peripheral regions than larger ones ().Citation79 In peripheral regions, the locations where Imp β1 was observed to be concentrated suggested that there are many available binding sites provided by the FG Nups. Due to the weak hydrophobic interactions between most FG Nups, they could form filamentous or porous structures, and the small gaps (or holes) between filaments would then allow smaller molecules to passively diffuse around these structures. When the molecular size of small molecules was reduced from 29 kDa to 0.3 kDa,Citation79 it was found that 0.3-kDa fluorescein molecules diffused approximately 2-fold farther into the peripheral filamentous regions than 29-kDa GFP molecules. Therefore, the distinct transport routes are not completely spatially separated. Moreover, it is reasonable to expect that smaller molecules, such as ions or water molecules, could diffuse farther, potentially extending across the entire peripheral region. On the other hand, the facilitated translocation of larger cargo complexes may occupy more space in the central channel simply due to the size of these complexes and, thus, cause more overlap between the two transport zones. Interestingly, experimental data indicate that it is unlikely that facilitated transport would completely occupy the central channel and block passive diffusion pathways in the center of NPCs under physiological conditions (unpublished data). It has been reported that very large cargo molecules require more transport receptors to assist their transit through a nuclear pore.Citation83-Citation85 Large numbers of transport receptors in a pore (termed a high pore occupancy) would induce conformational changes in FG filaments (), which would subsequently result in alteration of the overall configuration of the transport routes in the NPC.Citation79 Based on our preliminary data, we found that the FG filaments displayed different conformations under a low and high pore occupancy of Imp β1 in the NPC.Citation79 Some FG filaments were forced away from the axial center and toward their anchors on the walls of the pores. Conversely, other filaments extended into the center, stimulated by the change. Recently reported in vitro measurements suggested similar changes.Citation86 Such alterations of FG filaments have direct effects on the spatial geometry of the transport routesCitation79 and subsequently affect the kinetics of both passive and facilitated transport.Citation87 Moreover, the changes in these two pathways have been found to be closely correlated and nearly complementary to each other within nuclear pores.Citation79,Citation82 Therefore, distinct, but closely correlated transport routes for passive and facilitated transport exist in the NPC.
Figure 4. Diagrams showing the relative spatial locations of distinct transport routes in the NPC. (A) Spatially, signal-independent small molecules (golden particles) diffuse through an axial central conduit and exhibit slight overlap with the Imp β1 transport pathway (green cloud, a brighter color indicates a higher probability density) at their interface. (B) Smaller molecules (purple particles) diffuse farther into the Imp β1-occupied peripheral filamentous regions.
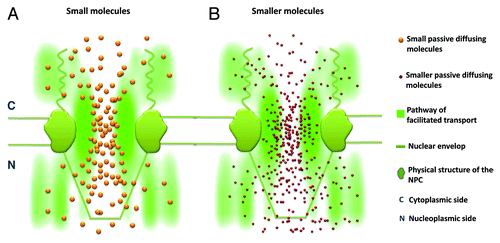
Figure 5. The conformational changes in the FG Nup filaments under low and high nuclear pore occupancies are demonstrated with simplified graphs. (A) The FG filaments (green curved lines) under a low pore occupancy of transport receptors (purple balls coated with green layers). (B) The status of FG filaments under a high pore occupancy.
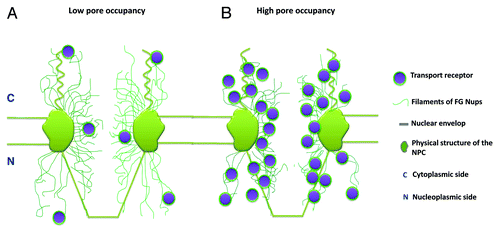
Data have shown that the functional influence between the passive and facilitated transport routes is unidirectional.Citation79 It has been reported that the transport kinetics and spatial transport routes involved in the passive diffusion of small molecules can be altered at a high Imp β1 pore occupancy (),Citation79,Citation87 but the presence of a high concentration of passively diffusing small molecules in the NPC has no effect on the nuclear transport of Imp β1.Citation79 The reason for this is that Imp β1 can bind to the FG Nups and induce conformational changes in the FG filaments in the NPC, while small passively diffusing molecules cannot. Small molecules do not display any interaction with the FG Nups and therefore cannot induce any changes in them. It is reasonable to infer that any molecules that can induce conformational changes in FG Nups will affect the status of both the passive diffusion and facilitated transport pathways both spatially and functionally.
In addition to the influence of the distinct passive and facilitated transport modes on each other, numerous reports have demonstrated the existence of competition within a given transport mode.Citation47-Citation51 The identification of transport routes for passive and facilitated transport will also help us understand this competition. The passive diffusion of various small molecules must occur through the same axial central channel in NPCs. When one molecule diffuses at a high flux or a very low efficiency, it could delay the diffusion of other molecules. In facilitated transport, competition is expected to occur between transport receptors that bind to the same sites on the FG Nups in the pores. These pre-occupied binding sites would cause inhibition of subsequent binding.
Finally, the 3D probability density maps obtained for both passive diffusion and transport receptor-facilitated transport further indicate the spatial distribution of FG filaments in the NPC.Citation79,Citation82 Driven by its interaction with FG filaments, Imp β1 can efficiently migrate through the NPC, despite the fact that its size is far above the cut-off threshold for passive diffusion. Studies have indicated that Imp β1 can interact with almost all of the FG Nups within the NPC.Citation27-Citation29 Therefore, the spatial pattern of Imp β1 localization in the NPC also reveals the location of the FG filaments, with a higher Imp β1 density suggesting a higher density of FG filaments. Thus, the low probability of observing Imp β1 in the axial central channel suggests that there are no, or very few, FG filaments in the central region.Citation82 As a result, the relatively less filamentous central conduit functions as the major diffusion path for the passive diffusion of signal-independent small molecules. Furthermore, the inhomogeneous spatial distribution of Imp β1 suggests that the spatial distribution of FG filaments along the NPC is not homogenous. Hence, these FG filaments likely cluster to form distinct sub-regions along the NPC,Citation81 which may provide unique micro-environments for specific biochemical interactions or multiple transport pathways for different cargo complexes, assisted by various transport receptors.
Conclusion and Perspective
Two core questions are closely related to the understanding of nuclear transport mechanisms. One is how passive and facilitated transport share the nuclear pore space, and the other concerns the structure of the selective barrier formed by the unstructured FG Nups in the NPC. Regarding the former, our tests involving various molecules (small molecules with a molecular weight of 0.3–40 kDa; transport receptors Imp β1, transportin, NTF2 and Tap/p15 and cargo complexes) have revealed that passive and facilitated transport exhibit distinct spatial transport routes that are not, however, completely separate either spatially or functionally.Citation79-Citation82 Moreover, the degree of spatial overlap and functional interference between these pathways depends on (1) the molecular size of small, passively diffusing molecules and facilitated cargo complexes and (2) the regulatory effect of the studied transport receptors on the FG Nups. Although further tests are still needed to examine the spatial transport routes of very small or very large molecules in transit in native NPCs, our expectation is that very small, signal-independent molecules, such as ions and water molecules, might be capable of diffusing across the entire FG filamentous region. Meanwhile, the facilitated transport of very large cargo complexes may extend into the axial central channel or involve conformational changes to allow these complexes to squeeze through the compact selective barrier. Concerning the latter question, the configuration of FG filaments in the pore may ultimately determine the geometry of transport routes for passive and facilitated transport in NPCs. Both the conformation of FG Nups and the geometry of transport routes can be further regulated by the pore occupancy of transport receptors to ultimately meet the trafficking needs of cells.Citation79 The inherently dynamic features of the FG Nups make them the ideal machinery for adopting various conformational changes and handling multiple tasks. The structure of FG Nups has recently been characterized via in vitro gel formation of extracted FG segmentsCitation41,Citation49,Citation50 and polarized microscopy determination of the orientations of the FG filaments.Citation88,Citation89 Novel experimental designs and new techniques are still urgently needed to ultimately structure the selective barrier in the NPC.
| Abbreviations: | ||
| NPC | = | nuclear pore complex |
| NE | = | nuclear envelope |
| Nups | = | nucleoporins |
| FG | = | phenylalanine-glycine |
| SPEED | = | single-point edge-excitation sub-diffraction |
Acknowledgments
This project was supported by grants from the National Institutes of Health (NIH GM094041 and GM097037).
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
References
- Hetzer MW. The nuclear envelope. Cold Spring Harb Perspect Biol 2010; 2:a000539; http://dx.doi.org/10.1101/cshperspect.a000539; PMID: 20300205
- Hetzer MW, Wente SR. Border control at the nucleus: biogenesis and organization of the nuclear membrane and pore complexes. Dev Cell 2009; 17:606 - 16; http://dx.doi.org/10.1016/j.devcel.2009.10.007; PMID: 19922866
- Powers MA, Forbes DJ. Nuclear transport: beginning to gel?. Curr Biol 2012; 22:R1006 - 9; http://dx.doi.org/10.1016/j.cub.2012.10.037; PMID: 23218007
- Görlich D, Kutay U. Transport between the cell nucleus and the cytoplasm. Annu Rev Cell Dev Biol 1999; 15:607 - 60; http://dx.doi.org/10.1146/annurev.cellbio.15.1.607; PMID: 10611974
- Carmody SR, Wente SR. mRNA nuclear export at a glance. J Cell Sci 2009; 122:1933 - 7; http://dx.doi.org/10.1242/jcs.041236; PMID: 19494120
- Speese SD, Ashley J, Jokhi V, Nunnari J, Barria R, Li Y, et al. Nuclear envelope budding enables large ribonucleoprotein particle export during synaptic Wnt signaling. Cell 2012; 149:832 - 46; http://dx.doi.org/10.1016/j.cell.2012.03.032; PMID: 22579286
- Raices M, D’Angelo MA. Nuclear pore complex composition: a new regulator of tissue-specific and developmental functions. Nat Rev Mol Cell Biol 2012; 13:687 - 99; http://dx.doi.org/10.1038/nrm3461; PMID: 23090414
- Tetenbaum-Novatt J, Rout MP. The mechanism of nucleocytoplasmic transport through the nuclear pore complex. Cold Spring Harb Symp Quant Biol 2010; 75:567 - 84; http://dx.doi.org/10.1101/sqb.2010.75.033; PMID: 21447814
- Maimon T, Medalia O. Perspective on the metazoan nuclear pore complex. Nucleus 2010; 1:383 - 6; PMID: 21326819
- Tu LC, Musser SM. Single molecule studies of nucleocytoplasmic transport. Biochim Biophys Acta 2011; 1813:1607 - 18; http://dx.doi.org/10.1016/j.bbamcr.2010.12.011; PMID: 21167872
- Görlich D, Mattaj IW. Nucleocytoplasmic transport. Science 1996; 271:1513 - 8; http://dx.doi.org/10.1126/science.271.5255.1513; PMID: 8599106
- Fahrenkrog B, Aebi U. The nuclear pore complex: nucleocytoplasmic transport and beyond. Nat Rev Mol Cell Biol 2003; 4:757 - 66; http://dx.doi.org/10.1038/nrm1230; PMID: 14570049
- Nigg EA. Nucleocytoplasmic transport: signals, mechanisms and regulation. Nature 1997; 386:779 - 87; http://dx.doi.org/10.1038/386779a0; PMID: 9126736
- Kuersten S, Ohno M, Mattaj IW. Nucleocytoplasmic transport: Ran, beta and beyond. Trends Cell Biol 2001; 11:497 - 503; http://dx.doi.org/10.1016/S0962-8924(01)02144-4; PMID: 11719056
- Cook A, Bono F, Jinek M, Conti E. Structural biology of nucleocytoplasmic transport. Annu Rev Biochem 2007; 76:647 - 71; http://dx.doi.org/10.1146/annurev.biochem.76.052705.161529; PMID: 17506639
- Weis K. Nucleocytoplasmic transport: cargo trafficking across the border. Curr Opin Cell Biol 2002; 14:328 - 35; http://dx.doi.org/10.1016/S0955-0674(02)00337-X; PMID: 12067655
- Weis K. Regulating access to the genome: nucleocytoplasmic transport throughout the cell cycle. Cell 2003; 112:441 - 51; http://dx.doi.org/10.1016/S0092-8674(03)00082-5; PMID: 12600309
- Fried H, Kutay U. Nucleocytoplasmic transport: taking an inventory. Cell Mol Life Sci 2003; 60:1659 - 88; http://dx.doi.org/10.1007/s00018-003-3070-3; PMID: 14504656
- Corbett AH, Silver PA. Nucleocytoplasmic transport of macromolecules. Microbiol Mol Biol Rev 1997; 61:193 - 211; PMID: 9184010
- Hoelz A, Debler EW, Blobel G. The structure of the nuclear pore complex. Annu Rev Biochem 2011; 80:613 - 43; http://dx.doi.org/10.1146/annurev-biochem-060109-151030; PMID: 21495847
- Rout MP, Blobel G. Isolation of the yeast nuclear pore complex. J Cell Biol 1993; 123:771 - 83; http://dx.doi.org/10.1083/jcb.123.4.771; PMID: 8227139
- Cronshaw JM, Krutchinsky AN, Zhang W, Chait BT, Matunis MJ. Proteomic analysis of the mammalian nuclear pore complex. J Cell Biol 2002; 158:915 - 27; http://dx.doi.org/10.1083/jcb.200206106; PMID: 12196509
- Rout MP, Aitchison JD. The nuclear pore complex as a transport machine. J Biol Chem 2001; 276:16593 - 6; http://dx.doi.org/10.1074/jbc.R100015200; PMID: 11283009
- Rout MP, Aitchison JD, Magnasco MO, Chait BT. Virtual gating and nuclear transport: the hole picture. Trends Cell Biol 2003; 13:622 - 8; http://dx.doi.org/10.1016/j.tcb.2003.10.007; PMID: 14624840
- Rout MP, Aitchison JD, Suprapto A, Hjertaas K, Zhao Y, Chait BT. The yeast nuclear pore complex: composition, architecture, and transport mechanism. J Cell Biol 2000; 148:635 - 51; http://dx.doi.org/10.1083/jcb.148.4.635; PMID: 10684247
- Suntharalingam M, Wente SR. Peering through the pore: nuclear pore complex structure, assembly, and function. Dev Cell 2003; 4:775 - 89; http://dx.doi.org/10.1016/S1534-5807(03)00162-X; PMID: 12791264
- Bayliss R, Littlewood T, Stewart M. Structural basis for the interaction between FxFG nucleoporin repeats and importin-beta in nuclear trafficking. Cell 2000; 102:99 - 108; http://dx.doi.org/10.1016/S0092-8674(00)00014-3; PMID: 10929717
- Bayliss R, Littlewood T, Strawn LA, Wente SR, Stewart M. GLFG and FxFG nucleoporins bind to overlapping sites on importin-beta. J Biol Chem 2002; 277:50597 - 606; http://dx.doi.org/10.1074/jbc.M209037200; PMID: 12372823
- Otsuka S, Iwasaka S, Yoneda Y, Takeyasu K, Yoshimura SH. Individual binding pockets of importin-beta for FG-nucleoporins have different binding properties and different sensitivities to RanGTP. Proc Natl Acad Sci U S A 2008; 105:16101 - 6; http://dx.doi.org/10.1073/pnas.0802647105; PMID: 18845677
- Rexach M, Blobel G. Protein import into nuclei: association and dissociation reactions involving transport substrate, transport factors, and nucleoporins. Cell 1995; 83:683 - 92; http://dx.doi.org/10.1016/0092-8674(95)90181-7; PMID: 8521485
- Ribbeck K, Lipowsky G, Kent HM, Stewart M, Görlich D. NTF2 mediates nuclear import of Ran. EMBO J 1998; 17:6587 - 98; http://dx.doi.org/10.1093/emboj/17.22.6587; PMID: 9822603
- Panté N, Kann M. Nuclear pore complex is able to transport macromolecules with diameters of about 39 nm. Mol Biol Cell 2002; 13:425 - 34; http://dx.doi.org/10.1091/mbc.01-06-0308; PMID: 11854401
- Talcott B, Moore MS. Getting across the nuclear pore complex. Trends Cell Biol 1999; 9:312 - 8; http://dx.doi.org/10.1016/S0962-8924(99)01608-6; PMID: 10407410
- Forbes DJ. Structure and function of the nuclear pore complex. Annu Rev Cell Biol 1992; 8:495 - 527; http://dx.doi.org/10.1146/annurev.cb.08.110192.002431; PMID: 1282353
- Allen TD, Cronshaw JM, Bagley S, Kiseleva E, Goldberg MW. The nuclear pore complex: mediator of translocation between nucleus and cytoplasm. J Cell Sci 2000; 113:1651 - 9; PMID: 10769196
- Denning DP, Patel SS, Uversky V, Fink AL, Rexach M. Disorder in the nuclear pore complex: the FG repeat regions of nucleoporins are natively unfolded. Proc Natl Acad Sci U S A 2003; 100:2450 - 5; http://dx.doi.org/10.1073/pnas.0437902100; PMID: 12604785
- Macara IG. Transport into and out of the nucleus. Microbiol Mol Biol Rev 2001; 65:570 - 94; http://dx.doi.org/10.1128/MMBR.65.4.570-594.2001; PMID: 11729264
- Peters R. Translocation through the nuclear pore complex: selectivity and speed by reduction-of-dimensionality. Traffic 2005; 6:421 - 7; http://dx.doi.org/10.1111/j.1600-0854.2005.00287.x; PMID: 15813752
- Lim RY, Fahrenkrog B, Köser J, Schwarz-Herion K, Deng J, Aebi U. Nanomechanical basis of selective gating by the nuclear pore complex. Science 2007; 318:640 - 3; http://dx.doi.org/10.1126/science.1145980; PMID: 17916694
- Ribbeck K, Görlich D. Kinetic analysis of translocation through nuclear pore complexes. EMBO J 2001; 20:1320 - 30; http://dx.doi.org/10.1093/emboj/20.6.1320; PMID: 11250898
- Frey S, Görlich D. A saturated FG-repeat hydrogel can reproduce the permeability properties of nuclear pore complexes. Cell 2007; 130:512 - 23; http://dx.doi.org/10.1016/j.cell.2007.06.024; PMID: 17693259
- Yamada J, Phillips JL, Patel S, Goldfien G, Calestagne-Morelli A, Huang H, et al. A bimodal distribution of two distinct categories of intrinsically disordered structures with separate functions in FG nucleoporins. Mol Cell Proteomics 2010; 9:2205 - 24; http://dx.doi.org/10.1074/mcp.M000035-MCP201; PMID: 20368288
- Akey CW, Radermacher M. Architecture of the Xenopus nuclear pore complex revealed by three-dimensional cryo-electron microscopy. J Cell Biol 1993; 122:1 - 19; http://dx.doi.org/10.1083/jcb.122.1.1; PMID: 8314837
- Hinshaw JE, Carragher BO, Milligan RA. Architecture and design of the nuclear pore complex. Cell 1992; 69:1133 - 41; http://dx.doi.org/10.1016/0092-8674(92)90635-P; PMID: 1617726
- Yang Q, Rout MP, Akey CW. Three-dimensional architecture of the isolated yeast nuclear pore complex: functional and evolutionary implications. Mol Cell 1998; 1:223 - 34; http://dx.doi.org/10.1016/S1097-2765(00)80023-4; PMID: 9659919
- Kramer A, Liashkovich I, Ludwig Y, Shahin V. Atomic force microscopy visualises a hydrophobic meshwork in the central channel of the nuclear pore. Pflugers Arch 2008; 456:155 - 62; http://dx.doi.org/10.1007/s00424-007-0396-y; PMID: 18060562
- Naim B, Brumfeld V, Kapon R, Kiss V, Nevo R, Reich Z. Passive and facilitated transport in nuclear pore complexes is largely uncoupled. J Biol Chem 2007; 282:3881 - 8; http://dx.doi.org/10.1074/jbc.M608329200; PMID: 17164246
- Feldherr CM, Akin D. The location of the transport gate in the nuclear pore complex. J Cell Sci 1997; 110:3065 - 70; PMID: 9365276
- Mohr D, Frey S, Fischer T, Güttler T, Görlich D. Characterisation of the passive permeability barrier of nuclear pore complexes. EMBO J 2009; 28:2541 - 53; http://dx.doi.org/10.1038/emboj.2009.200; PMID: 19680228
- Frey S, Görlich D. A saturated FG-repeat hydrogel can reproduce the permeability properties of nuclear pore complexes. Cell 2007; 130:512 - 23; http://dx.doi.org/10.1016/j.cell.2007.06.024; PMID: 17693259
- Fiserova J, Richards SA, Wente SR, Goldberg MW. Facilitated transport and diffusion take distinct spatial routes through the nuclear pore complex. J Cell Sci 2010; 123:2773 - 80; http://dx.doi.org/10.1242/jcs.070730; PMID: 20647373
- Hess HF, Betzig E, Harris TD, Pfeiffer LN, West KW. Near-field spectroscopy of the quantum constituents of a luminescent system. Science 1994; 264:1740 - 5; http://dx.doi.org/10.1126/science.264.5166.1740; PMID: 17839907
- Betzig E. Proposed method for molecular optical imaging. Opt Lett 1995; 20:237 - 9; http://dx.doi.org/10.1364/OL.20.000237; PMID: 19859146
- Patterson GH, Lippincott-Schwartz J. A photoactivatable GFP for selective photolabeling of proteins and cells. Science 2002; 297:1873 - 7; http://dx.doi.org/10.1126/science.1074952; PMID: 12228718
- Thompson RE, Larson DR, Webb WW. Precise nanometer localization analysis for individual fluorescent probes. Biophys J 2002; 82:2775 - 83; http://dx.doi.org/10.1016/S0006-3495(02)75618-X; PMID: 11964263
- Yildiz A, Forkey JN, McKinney SA, Ha T, Goldman YE, Selvin PR. Myosin V walks hand-over-hand: single fluorophore imaging with 1.5-nm localization. Science 2003; 300:2061 - 5; http://dx.doi.org/10.1126/science.1084398; PMID: 12791999
- Yildiz A, Park H, Safer D, Yang Z, Chen LQ, Selvin PR, et al. Myosin VI steps via a hand-over-hand mechanism with its lever arm undergoing fluctuations when attached to actin. J Biol Chem 2004; 279:37223; http://dx.doi.org/10.1074/jbc.C400252200; PMID: 15254036
- Fernández-Suárez M, Ting AY. Fluorescent probes for super-resolution imaging in living cells. Nat Rev Mol Cell Biol 2008; 9:929 - 43; http://dx.doi.org/10.1038/nrm2531; PMID: 19002208
- Lippincott-Schwartz J, Patterson GH. Photoactivatable fluorescent proteins for diffraction-limited and super-resolution imaging. Trends Cell Biol 2009; 19:555 - 65; http://dx.doi.org/10.1016/j.tcb.2009.09.003; PMID: 19836954
- Rust MJ, Bates M, Zhuang X. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM). Nat Methods 2006; 3:793 - 5; http://dx.doi.org/10.1038/nmeth929; PMID: 16896339
- Betzig E, Patterson GH, Sougrat R, Lindwasser OW, Olenych S, Bonifacino JS, et al. Imaging intracellular fluorescent proteins at nanometer resolution. Science 2006; 313:1642 - 5; http://dx.doi.org/10.1126/science.1127344; PMID: 16902090
- Hess ST, Girirajan TP, Mason MD. Ultra-high resolution imaging by fluorescence photoactivation localization microscopy. Biophys J 2006; 91:4258 - 72; http://dx.doi.org/10.1529/biophysj.106.091116; PMID: 16980368
- Huang B, Bates M, Zhuang X. Super-resolution fluorescence microscopy. Annu Rev Biochem 2009; 78:993 - 1016; http://dx.doi.org/10.1146/annurev.biochem.77.061906.092014; PMID: 19489737
- Patterson G, Davidson M, Manley S, Lippincott-Schwartz J. Superresolution imaging using single-molecule localization. Annu Rev Phys Chem 2010; 61:345 - 67; http://dx.doi.org/10.1146/annurev.physchem.012809.103444; PMID: 20055680
- Huang B, Wang W, Bates M, Zhuang X. Three-dimensional super-resolution imaging by stochastic optical reconstruction microscopy. Science 2008; 319:810 - 3; http://dx.doi.org/10.1126/science.1153529; PMID: 18174397
- Akey CW. Interactions and structure of the nuclear pore complex revealed by cryo-electron microscopy. J Cell Biol 1989; 109:955 - 70; http://dx.doi.org/10.1083/jcb.109.3.955; PMID: 2768344
- Akey CW, Radermacher M. Architecture of the Xenopus nuclear pore complex revealed by three-dimensional cryo-electron microscopy. J Cell Biol 1993; 122:1 - 19; http://dx.doi.org/10.1083/jcb.122.1.1; PMID: 8314837
- Beck M, Lucić V, Förster F, Baumeister W, Medalia O. Snapshots of nuclear pore complexes in action captured by cryo-electron tomography. Nature 2007; 449:611 - 5; http://dx.doi.org/10.1038/nature06170; PMID: 17851530
- Beck M, Förster F, Ecke M, Plitzko JM, Melchior F, Gerisch G, et al. Nuclear pore complex structure and dynamics revealed by cryoelectron tomography. Science 2004; 306:1387 - 90; http://dx.doi.org/10.1126/science.1104808; PMID: 15514115
- Shtengel G, Galbraith JA, Galbraith CG, Lippincott-Schwartz J, Gillette JM, Manley S, et al. Interferometric fluorescent super-resolution microscopy resolves 3D cellular ultrastructure. Proc Natl Acad Sci U S A 2009; 106:3125 - 30; http://dx.doi.org/10.1073/pnas.0813131106; PMID: 19202073
- Sun Y, McKenna JD, Murray JM, Ostap EM, Goldman YE. Parallax: high accuracy three-dimensional single molecule tracking using split images. Nano Lett 2009; 9:2676 - 82; http://dx.doi.org/10.1021/nl901129j; PMID: 19496608
- Toprak E, Balci H, Blehm BH, Selvin PR. Three-dimensional particle tracking via bifocal imaging. Nano Lett 2007; 7:2043 - 5; http://dx.doi.org/10.1021/nl0709120; PMID: 17583964
- Dupont A, Lamb DC. Nanoscale three-dimensional single particle tracking. Nanoscale 2011; 3:4532 - 41; http://dx.doi.org/10.1039/c1nr10989h; PMID: 21960183
- Ram S, Prabhat P, Chao J, Ward ES, Ober RJ. High accuracy 3D quantum dot tracking with multifocal plane microscopy for the study of fast intracellular dynamics in live cells. Biophys J 2008; 95:6025 - 43; http://dx.doi.org/10.1529/biophysj.108.140392; PMID: 18835896
- Tang J, Akerboom J, Vaziri A, Looger LL, Shank CV. Near-isotropic 3D optical nanoscopy with photon-limited chromophores. Proc Natl Acad Sci U S A 2010; 107:10068 - 73; http://dx.doi.org/10.1073/pnas.1004899107; PMID: 20472826
- Peters R. The nanopore connection to cell membrane unitary permeability. Traffic 2005; 6:199 - 204; http://dx.doi.org/10.1111/j.1600-0854.2005.00269.x; PMID: 15702988
- Jovanovic-Talisman T, Tetenbaum-Novatt J, McKenney AS, Zilman A, Peters R, Rout MP, et al. Artificial nanopores that mimic the transport selectivity of the nuclear pore complex. Nature 2009; 457:1023 - 7; http://dx.doi.org/10.1038/nature07600; PMID: 19098896
- Kowalczyk SW, Kapinos L, Blosser TR, Magalhães T, van Nies P, Lim RY, et al. Single-molecule transport across an individual biomimetic nuclear pore complex. Nat Nanotechnol 2011; 6:433 - 8; http://dx.doi.org/10.1038/nnano.2011.88; PMID: 21685911
- Ma J, Goryaynov A, Sarma A, Yang W. Self-regulated viscous channel in the nuclear pore complex. Proc Natl Acad Sci U S A 2012; 109:7326 - 31; http://dx.doi.org/10.1073/pnas.1201724109; PMID: 22529346
- Goryaynov A, Ma J, Yang W. Single-molecule studies of nuclear transport: from one dimension to three dimensions. Integr Biol 2012; 4:10 - 21; http://dx.doi.org/10.1039/c1ib00041a
- Yang W. ‘Natively unfolded’ nucleoporins in nucleocytoplasmic transport: clustered or evenly distributed?. Nucleus 2011; 2:10 - 6; http://dx.doi.org/10.4161/nucl.2.1.13818; PMID: 21647294
- Ma J, Yang W. Three-dimensional distribution of transient interactions in the nuclear pore complex obtained from single-molecule snapshots. Proc Natl Acad Sci U S A 2010; 107:7305 - 10; http://dx.doi.org/10.1073/pnas.0908269107; PMID: 20368455
- Bednenko J, Cingolani G, Gerace L. Nucleocytoplasmic transport: navigating the channel. Traffic 2003; 4:127 - 35; http://dx.doi.org/10.1034/j.1600-0854.2003.00109.x; PMID: 12656985
- Lowe AR, Siegel JJ, Kalab P, Siu M, Weis K, Liphardt JT. Selectivity mechanism of the nuclear pore complex characterized by single cargo tracking. Nature 2010; 467:600 - 3; http://dx.doi.org/10.1038/nature09285; PMID: 20811366
- Ribbeck K, Görlich D. The permeability barrier of nuclear pore complexes appears to operate via hydrophobic exclusion. EMBO J 2002; 21:2664 - 71; http://dx.doi.org/10.1093/emboj/21.11.2664; PMID: 12032079
- Schoch RL, Kapinos LE, Lim RY. Nuclear transport receptor binding avidity triggers a self-healing collapse transition in FG-nucleoporin molecular brushes. Proc Natl Acad Sci U S A 2012; 109:16911 - 6; http://dx.doi.org/10.1073/pnas.1208440109; PMID: 23043112
- Yang W, Musser SM. Nuclear import time and transport efficiency depend on importin beta concentration. J Cell Biol 2006; 174:951 - 61; http://dx.doi.org/10.1083/jcb.200605053; PMID: 16982803
- Kampmann M, Atkinson CE, Mattheyses AL, Simon SM. Mapping the orientation of nuclear pore proteins in living cells with polarized fluorescence microscopy. Nat Struct Mol Biol 2011; 18:643 - 9; http://dx.doi.org/10.1038/nsmb.2056; PMID: 21499242
- Mattheyses AL, Kampmann M, Atkinson CE, Simon SM. Fluorescence anisotropy reveals order and disorder of protein domains in the nuclear pore complex. Biophys J 2010; 99:1706 - 17; http://dx.doi.org/10.1016/j.bpj.2010.06.075; PMID: 20858414
- Tokunaga M, Imamoto N, Sakata-Sogawa K. Highly inclined thin illumination enables clear single-molecule imaging in cells. Nat Methods 2008; 5:159 - 61; http://dx.doi.org/10.1038/nmeth1171; PMID: 18176568