Abstract
The nuclear lamina guards the genome and in many ways contributes to regulating nuclear function. Increasing evidence indicates that the lamina dynamically interacts with chromatin mainly through large repressive domains, and recent data suggest that at least some of the lamin-genome contacts may be developmentally significant. In an attempt to provide an additional meaning to lamin-genome contacts, a recent study characterized the association of gene promoters with A-type lamins in progenitor and differentiated cells. Here, we discuss how A-type lamins interact with spatially defined promoter regions, and the relationship between these interactions, associated chromatin marks and gene expression outputs. We discuss the impact of A-type lamins on nucleus-wide and local chromatin organization. We also address how lamin-promoter interactions are redistributed during differentiation of adipocyte progenitors into adipocytes. Finally, we propose a model of lineage-specific “unlocking” of developmentally regulated loci and its significance in cellular differentiation.
Nuclear Lamins and their Connections to the Genome
The nuclear envelope and its associated components contribute to defining position, shape and functions of the eukaryotic cell nucleus.Citation1 The nuclear envelope consists of an outer and inner nuclear membrane perforated by nuclear pores and underlined by an intermediate filament meshwork called the nuclear lamina (NL).Citation1-Citation4 Two types of nuclear lamins make up the somatic cell lamina: A-types lamins (lamins A and C, often referred to as lamin A/C or LMNA), splice products of the LMNA gene, and B-type lamins, consisting of lamins B1 and B2 encoded by the LMNB1 and LMNB2 genes. B-type lamins are constitutively expressed, and are anchored to the inner nuclear membrane through a farnesylated CAXX motif in their C-terminus.Citation4 In contrast, A-type lamins are developmentally regulated: they are undetectable in early embryos or certain blast cells but are expressed in more differentiated progenitors and in terminally differentiated cells,Citation4-Citation6 with a few exceptions.Citation7 As part of their proteolytic processing, mature A-type lamins lose the C-terminal farnelysation site of pre-lamin A, and therefore do not anchor directly into the inner nuclear membrane.Citation4 Rather, they associate with B-type lamins in the peripheral NL,Citation8 and a pool of lamin A/C is nucleoplasmic and detergent-soluble.Citation9 There is to date surprisingly little information on whether A- and B-type lamins play distinct orcommon roles in development, whether peripheral and nucleoplasmic pools of A-type lamins have different functions, and how these pools are regulated.
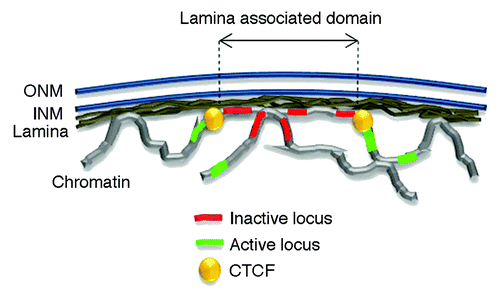
Presumably by virtue of their ability to bind DNA and nucleosomes in vitro,Citation10 A- and B-type lamins establish thousands of contacts with chromatin.Citation11 These occur through large lamina-associated domains (LADs) spanning 0.1 to 10 megabases (Mb) of linear genomeCitation11-Citation19 (). LADs are generally conserved between cell types although some cell type-specificity is observed.Citation20 Lamin-genome contacts can also be more spatially restricted and occur on clustered or stand-alone loci.Citation13,Citation21,Citation22
Figure 1. The nuclear lamina interacts with the genome through lamina-associated domains or LADs. The nuclear envelope consists of an outer and inner nuclear membrane (ONM and INM respectively) under laid by the lamina. LADs largely consist of inactive chromatin region and are bordered by CTCF proteins. Active loci preferentially locate outside LADs.
Lamins have been proposed to play a role in the organization of heterochromatin7,17‑19,22‑24 and to influence the 3-dimensional (3D) conformation of the genome.Citation16 Lamins also tether signaling molecules and transcription factors,Citation25-Citation27 and indirectly connect the nuclear interior with elements of the cytoskeleton,Citation3,Citation4 influencing nuclear positioning within the cell.Citation4,Citation28 It does not come as a surprise, then, that mutations in nuclear lamins and LMNA in particular, cause diseases. The co-called laminopathies present various symptoms such as partial lipodystrophies, myodystrophies, cardio-myopathies, skeletal abnormalities, neuropathies or premature aging.Citation2,Citation27,Citation29 The molecular mechanisms leading to laminopathies remain unclear but they may involve abnormalities in heterochromatin organization,Citation30 signal transductionCitation27 and autophagy.Citation31
An emerging hypothesis is that through their extensive range of interaction partners, lamins may relay signals from the cytoplasm to chromatin, and contribute to modulating gene expression and cellular functions.Citation32 This implies that at least some of the lamin-genome contacts are locus-specific and regulated in a temporal and cell type-specific fashion. Recent data,Citation14,Citation18,Citation24 including some from our laboratory,Citation22 suggests that this may be the case. It is also conceivable that actively transcribing domains are pulled away from the NL toward the nuclear interior, while silent domains randomly contact the NL and become “locked” at the NL. Recent single-cell imaging of LADs argues for some stochasticity in the formation of LADs.Citation17
Here, we highlight recent insights on the association of nuclear lamins with the genome, including gene promoters. Topological maps of promoter-associated LMNA in relation to chromatin modifications refine the concept of LADs and point to an unsuspected modulatory role of lamins on the transcription outcome of genes they associate with. In addition, perturbations in NL composition by downregulating A-type lamins show an unsuspected influence of LMNA loss on chromatin organization at the genome-wide level and at the level of individual promoters. Lastly, differentiation redistributes lamin-promoter contacts with a degree of lineage specificity. This suggest a model of lineage-specific “unlocking” of genes from the nuclear lamina as a priming step for differentiation-induced transcriptional activation.
LMNA-interacting Loci Overall Cluster in Repressive Chromatin
LADs generally reside in transcriptionally repressive chromatin domains and accordingly, they are overall gene-poor or contain genes which are silent or expressed at low level.Citation12,Citation13,Citation33-Citation35 Yet, not all genes located within LADs or interacting with lamins are repressed,Citation13 suggesting a sub-LAD tuning of gene expression. An examination of this issue recently prompted the question of whether lamin would interact with gene regulatory elements such as promoters, the chromatin environment of these promoters, the expression status of the corresponding genes, and whether lamin-promoter interactions are developmentally regulated.Citation22
An interrogation of LMNA contacts with promoters by chromatin immunoprecipitation (ChIP) in human adipose stem cells (ASCsCitation36), the precursors of adipocytes, identified over 4000 promoters in contact with LMNA.Citation22 Interacting sequences are enriched in GAGA motifs, consistent with mouse data,Citation35 and in A/T-rich stretches. Whether these interactions are mediated by transcription factors remain unknown but is likely,Citation35 and some of these factors may potentially link LMNA-promoter contacts to developmental regulation.Citation37,Citation38
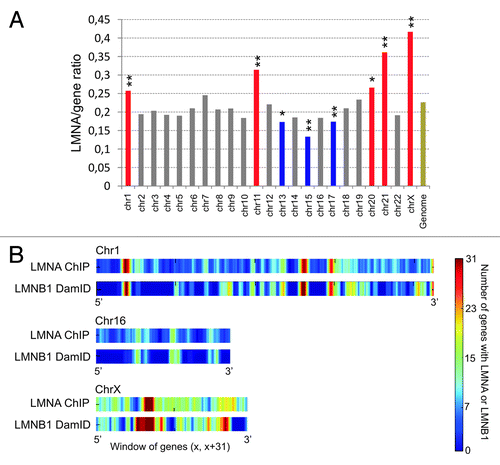
A feature of LMNA, in contrast to B-type lamins, is their existence as a nucleoplasmic pool, in addition to their association with the NL.Citation9 Accordingly, all chromosomes harbor some level of LMNA enrichment, with chromosomes 1, 11, 21, and X being highly enriched (). There is however no evidence of LMNA ‘hot spots’ at or near known genomic landmarks such as pericentric or subtelomeric regions.Citation22 However, alternating windows of contiguous LMNA-rich and LMNA-poor genes can be evidenced across the genome, consistent with a view of lamin‑associated regions at the nuclear periphery as well as in the nuclear interior ().Citation22 Incidentally, we find a strong correlation between LMNA enriched domains identified by ChIP in ASCs,Citation22 and LMNB1 LADs identified by DamID in lung fibroblastsCitation13 (). This confirms the view that lamin-rich domains are in majority conserved between cell types,Citation20 even when they are identified by different methods.
Figure 2. Genes with a promoter interacting with LMNA form clusters in the genome. (A) Chromosome distribution of LMNA enrichment determined by the ratio of LMNA-interacting genes to all genes per chromosome. Red bars indicate LMNA enrichment, and blue bars indicate LMNA impoverishment, both relative to genome-average LMNA occupancy (Genome; **P < 10−4; *P < 10−2; Chi-square). The data are from our laboratory (Lund EG and Collas P, unpublished). (B) Genes with a promoter enriched in lamin A cluster into “lamin-rich domains”.Citation22 Graph shows LMNA enrichment in 31-gene windows across chromosomes 1, 16, and X in human ASCs, determined by ChiP. For comparison, domains of LMNB1 enrichment identified by DamIDCitation13 in lung fibroblasts are also shown.
The genome-wide patterns of LADs identified by DamID for A- and B-type lamins are also similar,Citation17,Citation20 despite the exclusive peripheral localization of LMNB1 (in contrast to LMNA which is also found in the nuclear interior). This conundrum may be solved by several observations: (1) differences do exist between A- and B-type lamin LADs; (2) the nuclear envelope invaginates into the nuclear interior,Citation39,Citation40 which may result in LMNB1 contacting internal chromosomes; (3) it remains formally possible that intranuclear LMNA does not significantly associate with chromatin, and that LMNA LADsCitation17,Citation20 or lamin-rich domainsCitation22 are largely peripheral. Clearly, interactions of peripheral vs. internal A-type lamins with chromatin remain to be examined closely.
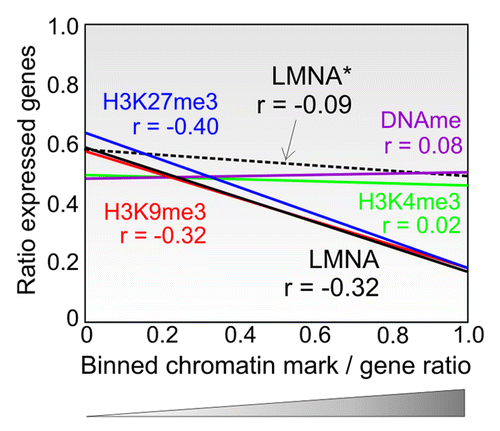
What is the relationship between lamin enrichment and chromatin activity? In all cell types examined to date, LADs consistently lie in repressive chromatin environments. Similarly, gene expression in a genomic ‘neighborhood’ (e.g., a genomic bin of 1 Mb) negatively correlates with LMNA enrichment in that neighborhoodCitation22 (, continuous black line). However, in a neighborhood of LMNA (i.e., a genomic area with many genes interacting with LMNA), the expression of genes which are themselves not bound by LMNA shows no relationship to LMNA enrichment in their neighborhood (, dotted black line). Thus, localization of a gene in a LMNA-rich neighborhood is by itself not repressive, suggesting that a LMNA neighborhood is in itself not conducive of transcriptional repression. Moreover, a subset (~2%) of LMNA-bound promoters harbors trimethylated H3K4me3 (H4K4me3),Citation22 which marks the TSS of active or potentially active genes.Citation41 These genes show a wide range of expression levels arguing that a subset of LMNA-bound genes escapes transcriptional repression.Citation22 So association of a promoter with LMNA is not systematically linked to transcriptional repression, implying that LMNA interaction by itself is not conducive of a repressive state. LMNA may in itself not have any repressive function but may be involved in genomic recruitment to, or stabilization within, a repressive chromatin compartment.Citation17
Figure 3. Genome-wide context of LMNA interaction with promoters shows association with a repressive environment. LMNA enrichment in a genomic ‘neighborhood’ affects the expression of genes associated with LMNA (black line) but not the expression of genes not interacting with LMNA in that neighborhood (dotted line; LMNA*). The data show regression lines and Spearman correlations (r). X-axis indicates ratios of genes with a given mark (LMNA, H3K4me3, H3K9me3, H3K27me3 or DNA methylation) to that of all genes in 1 Mb bins (bin data points are not shown). Y-axis indicates the ratios of expressed genes to all genes in a bin. A bin is assimilated to a ‘neighborhood’). Data for LMNA are from RefCitation22; data for chromatin modifications are from our laboratory (Lund EG and Collas P, unpublished data).
Conceptually, peripheral and intranuclear lamins may be associated with distinct transcriptional functions. At the nuclear periphery, lamins may be involved in the concentration of repressive chromatin modifiers such as histone methyltransferases and deacetylases, and other co-repressors.Citation12,Citation15,Citation33,Citation34,Citation42-Citation44 The NL may also tether chromatin at the nuclear periphery as a consequence of repressive histone marking. Notwithstanding, inner nuclear membrane proteins which interact with chromosomes, such as LBR, MAN1, or LAP2βCitation45-Citation47 may also be involved in these functions. Intranuclear lamins may locally tether genomic sites within the nuclear interior for distinct transcriptional regulatory functions, not all of which may be repressive. These speculations remain to be tested.
Topological Landscape of LMNA on Promoters
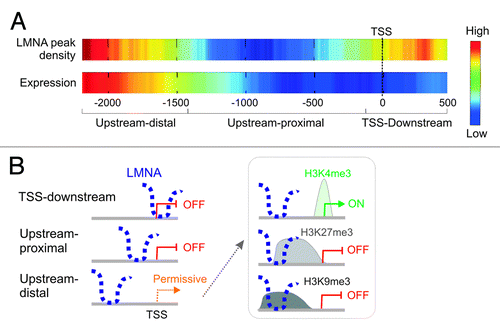
The chromatin context of LADs begins to be well characterized,Citation11 even at the single-cell level.Citation17 Nonetheless the recent data topologically refine and extend the concept of LADs in several ways.Citation22 First, results reveal LMNA association with spatially restricted regions on promoters, with a median size of 1.5 ± 0.7 Kb. This is consistent with, in mouse cells, LADs harboring many confined lamina-associating sequences in the Kb range,Citation35 and with focal genomic domains associated with the inner nuclear membrane in Caenorhabditis elegans.Citation48 Second, LMNA occupancy is not uniform within promoter regions: LMNA density is highest in an “upstream-distal” region, and at the TSS and 5′ end of genes; less frequently, LMNA occupies a TSS upstream-proximal region (). Interestingly, LMNA enrichment in the various sub-regions is linked to distinct gene ontology terms (), supporting the finding that the incidence of promoters enriched in LMNA in more than one sub-region is minimal.Citation22
Figure 4. Topological landscape of LMNA on promoters. (A) LMNA peak density on promoter regions. Highest densities are found upstream-distal from the TSS, and in a TSS-downstream region. (B) Genes with a promoter bound to LMNA in the sub-regions defined from the density map fall into distinct gene ontology categories. (C) Distribution of LMNA peaks over 4 Kb of promoter regions in adipose stem cells. Promoters with a LMNA peak spanning the 5′ end of tiled regions or entirely contained within the tile regions are the most frequent. Numbers of genes in each cluster are shown (Lund EG and Collas P, unpublished).
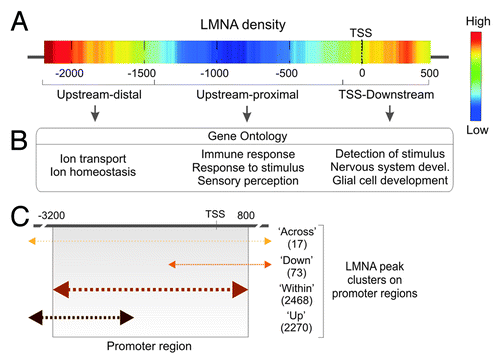
These observations do not preclude LMNA enrichment beyond the relatively narrow promoter regions examined in that study.Citation22 In fact, four clusters of LMNA peaks can be identified based on their topology over promoter regions (): an “up-cluster” spanning the 5′ end of tiled regions, a “within-cluster” restricted to the 4 Kb region, a “down-cluster” spanning the 3′ end of tiled regions, and an “across-cluster” crossing both 5′ and 3′ ends. This clustering indicates that LMNA generally interacts with distinct spatially restricted promoter sub-regions.
LMNA Positioning on a Promoter Is Linked to a Gene Expression Outcome
Functional insights on this differential promoter marking by LMNA begin to emerge from expression data. Positioning of LMNA on a promoter appears to be associated with a distinct expression pattern ().Citation22 Upstream-distal LMNA interaction is compatible with gene expression depending on the associated histone modifications; in contrast, upstream-proximal and TSS-downstream association correlates with gene inactivity (). This repression is independent of H3K4me3, H3K9me3, H3K27me3,Citation22 or DNA methylation (Lund EG and Collas P, unpublished observations). Notably, TSS occupancy by LMNA correlates with a silent state even when H3K4me3 marks the TSS. This may be due to inhibition of nucleosome turnover,Citation49 recruitment of repressive chromatin modifiers,Citation43 or prevention of TSS occupancy by RNA polymerase II (RNAPII). Consistent with this possibility, we find little overlap between LMNA enrichment and RNAPII occupancy, while depletion of LMNA from the TSS after LMNA downregulation correlates with enhanced RNAPII density at this site (Lund EG, Anja Oldenburg, and Collas P, unpublished data). This raises the possibility that LMNA may constitute a barrier to assembly of the transcription machinery at the TSS.
Figure 5. Positioning of lamin A on promoters correlates with the expression output of the corresponding gene. (A) LMNA positioning over the TSS or downstream, of proximal upstream of the TSS, is associated with absence of expression, whereas localization upstream-distal is permissive. Note that the high-low scale is different for LMNA and expression heat maps in that despite transcriptional permissiveness, the frequency of expressed genes with LMNA upstream-distal of the TSS remains low. (B) Schematic representation of the relationship between LMNA positioning and gene expression. The permissive state is detailed on the right (box) and depends on the associated histone modification.
LMNA Influences Nucleus-wide and Local Chromatin Marks
The structural role of the NL and its interaction with the genome suggest that the lamina plays a role in organizing chromatin into domains marked by distinct epigenetic modifications. In line with this possibility, downregulation of LMNA with an shRNA impacts the nature of histone modifications on promoters, by lowering the incidence of repressive modifications (e.g., H3K9me3 or H3K27me3) and strongly increasing permissive H3K4me3 marking.Citation22 Notwithstanding, loss of LMNA does not correlate with gene activation.Citation22 Moreover, the large numbers of genes whose histone modification pattern is altered by LMNA loss, compared with those initially marked by LMNA, implies that these large scale chromatin changes affect loci not associated with LMNA to start with. This argues that LMNA influences chromatin composition beyond sites it interacts with.
In addition to a genome-scale influence, the effect of downregulating LMNA is also perceived locally, at the sub-promoter level.Citation22 We found that LMNA downregulation, but not full depletion, results in some promoters retaining LMNA. On these promoters, LMNA shows a strong tendency to be delocalized from the TSS. This, in turn, affects the enrichment and positioning of histone modifications: whereas densities of H3K9me3 or H3K27me3 on those promoters that retain LMNA are not affected, H3K4me3 marking extends into the promoter area, over ~6 nucleosomes upstream of the TSS.Citation22 This intriguingly occurs on all H3K4me3-marked promoters, i.e., not only on promoters that harbored LMNA at the TSS to start with and that lost it upon LMNA knock-down, but also on promoters that did not associate with LMNA to begin with. This observation reinforces the long-range influence of LMNA on chromatin organization at the nucleus-wide level.
Adipogenic Differentiation Remodels LMNA-Promoter Interactions
Nuclear lamins have been involved in the formation and maintenance of organs and tissues but there is still little understanding of their role in these processes. The Drosophila B-type lamin regulates epidermal growth factor receptor signaling in cyst stem cells in the niche supporting male germ cell development, by regulating the distribution of specific nucleoporins within nuclear pores.Citation50 A sequential role of LBR and LMNA in tethering heterochromatin at the nuclear periphery during differentiation has also been reported, showing how changes in the nuclear envelope contribute to positioning heterochromatin and altering gene expression and differentiation.Citation7 This argues for a role of lamin-genome contacts, and particularly of changes in these contacts, during differentiation.
As a first step to addressing a putative role of lamins in differentiation, embryonic stem (ES) cell differentiation into neuronal progenitors (step 1) followed by astrocytic differentiation (step 2) revealed that loci that dissociated from the NL (B-type lamins) during step 1 had a stronger propensity to be activated during step 2 than those that retained NL association.Citation14 What remains unknown in that study however is whether upregulation of LMNA when ES cells exit pluripotency (step 1) plays a role in maintaining loci inactive at this stage after dissociation from B-type lamins. LMNA expression may conceivably provide an additional level of regulation of developmental gene induction: genes destined to be activated upon terminal differentiation may be handed over to LMNA at the progenitor stage, and terminal differentiation may dissociate these genes from LMNA before activation.Citation51
Recent data argue indeed for a release of lineage-specific genes from LMNA upon terminal differentiation.Citation22,Citation51 Differentiation of adipocyte progenitors (ASCs) into adipocytes is initiated by changes in epigenetic and transcription factor binding statesCitation52,Citation53 and results in profound remodeling of the nucleus. It is also accompanied by remodeling of LMNA-genome interactions: in ASCs: losses of and gains of LMNA peaks occur on many promoters involved in developmental, signaling, metabolic, chromatin remodeling and RNA processing functions.Citation22 In particular, genes controlling adipogenic induction, which do not interact with LMNA in ASCs (e.g., PPARG, FABP4, LEP), dissociate from LMNA in adipocytes (). Moreover, these acquire H3K4me3 () and become expressed in adipocytes.Citation52 In contrast, genes implicated in differentiation into other lineages (e.g., MYOG) or involved in pluripotency (i.e., active in ES cells but not in ASCs or adipocytes; e.g., POU5F1), and which interact with LMNA in ASCs, remain associated with LMNA in adipocytes ().
Figure 6. Lineage-specific unlocking genes for transcriptional activation. (A) Examples of the lineage-specificity of the dissociation of promoters from the lamina (here, LMNA) and acquisition of H3K4me3, upon differentiation of adipocyte progenitors (ASCs) into adipocytes. The P2 promoter (but not P1) of the PPARG gene, which is activated after adipogenic induction, displays a loss of LMNA peak (enrichment) in adipocytes (red arrow), and gains an H3K4me3 peak (green arrowhead). In contrast, promoters of the myogenic gene MYOG and of the pluripotency gene POU5F1 retain LMNA peaks (blue arrows) and do not acquire H3K4me3. GAPDH, which is expressed in both ASCs and adipocytes, is not associated with LMNA and displays a peak of H3K4me3 in both cell types (blue closed arrows). Horizontal bars indicate the presence and position of peaks of LMNA or H3K4me3 enrichment on promoters. (B) Working model of the lineage specificity of gene unlocking from LMNA during differentiation of progenitor cells into distinct lineages. Genes required for differentiation into a given lineage “A” or “B” and which interact with LMNA in progenitor cells (e.g., mesenchymal stem cells) become “unlocked”, while those that are not required for differentiation remain associated with LMNA.
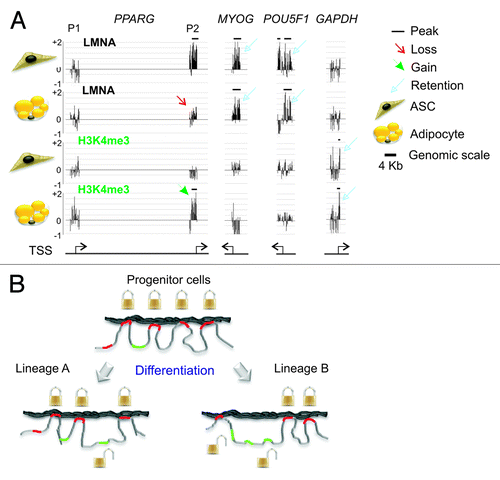
Could this unlocking of loci from the NL be lineage-dependent? This would be an attractive possibility: it seems that lamin-linked loci controlling induction into a given lineage are released from the lamina, while those controlling other lineages remain bound ().Citation51 Disengagement from nuclear lamins may create a promoter conformation enabling acquisition of permissive chromatin modifications and RNAPII loading onto the TSS. How LMNA displacement may promote RNAPII loading is not known. Interestingly, a recent report on how enhancer RNAs enable chromatin access to RNAPII at define sites, including TSSs, and apparently in a lineage-specific manner,Citation54 raises the hypothesis of an interplay between LMNA and non-coding RNAs on the regulation of expression of lineage-specific genes. The advent of powerful genomic tools to investigate 3D genome architecture,Citation11,Citation22 together with no less insightful single-cell approaches,Citation17,Citation55 should rapidly enhance our understanding of the regulation of genome architecture during differentiation and development.
| Abbreviations: | ||
| ChIP | = | chromatin immunoprecipitation |
| ES cell | = | embryonic stem cell |
| LAD | = | lamina associated domain |
| LMNA | = | lamin A/C |
| Mb | = | megabase |
| NL | = | nuclear lamina |
| RNAPII | = | RNA polymerase II |
| TSS | = | transcription start site |
References
- Dechat T, Gesson K, Foisner R. Lamina-independent lamins in the nuclear interior serve important functions. Cold Spring Harb Symp Quant Biol 2010; 75:533 - 43; http://dx.doi.org/10.1101/sqb.2010.75.018; PMID: 21209392
- Butin-Israeli V, Adam SA, Goldman AE, Goldman RD. Nuclear lamin functions and disease. Trends Genet 2012; 28:464 - 71; http://dx.doi.org/10.1016/j.tig.2012.06.001; PMID: 22795640
- Gerace L, Huber MD. Nuclear lamina at the crossroads of the cytoplasm and nucleus. J Struct Biol 2012; 177:24 - 31; http://dx.doi.org/10.1016/j.jsb.2011.11.007; PMID: 22126840
- Burke B, Stewart CL. The nuclear lamins: flexibility in function. Nat Rev Mol Cell Biol 2013; 14:13 - 24; http://dx.doi.org/10.1038/nrm3488; PMID: 23212477
- Stewart C, Burke B. Teratocarcinoma stem cells and early mouse embryos contain only a single major lamin polypeptide closely resembling lamin B. Cell 1987; 51:383 - 92; http://dx.doi.org/10.1016/0092-8674(87)90634-9; PMID: 3311384
- Worman HJ, Lazaridis I, Georgatos SD. Nuclear lamina heterogeneity in mammalian cells. Differential expression of the major lamins and variations in lamin B phosphorylation. J Biol Chem 1988; 263:12135 - 41; PMID: 3403563
- Solovei I, Wang AS, Thanisch K, Schmidt CS, Krebs S, Zwerger M, Cohen TV, Devys D, Foisner R, Peichl L, et al. LBR and lamin A/C sequentially tether peripheral heterochromatin and inversely regulate differentiation. Cell 2013; 152:584 - 98; http://dx.doi.org/10.1016/j.cell.2013.01.009; PMID: 23374351
- Delbarre E, Tramier M, Coppey-Moisan M, Gaillard C, Courvalin JC, Buendia B. The truncated prelamin A in Hutchinson-Gilford progeria syndrome alters segregation of A-type and B-type lamin homopolymers. Hum Mol Genet 2006; 15:1113 - 22; http://dx.doi.org/10.1093/hmg/ddl026; PMID: 16481358
- Kolb T, Maass K, Hergt M, Aebi U, Herrmann H. Lamin A and lamin C form homodimers and coexist in higher complex forms both in the nucleoplasmic fraction and in the lamina of cultured human cells. Nucleus 2011; 2:425 - 33; http://dx.doi.org/10.4161/nucl.2.5.17765; PMID: 22033280
- Stierlé V, Couprie J, Ostlund C, Krimm I, Zinn-Justin S, Hossenlopp P, Worman HJ, Courvalin JC, Duband-Goulet I. The carboxyl-terminal region common to lamins A and C contains a DNA binding domain. Biochemistry 2003; 42:4819 - 28; http://dx.doi.org/10.1021/bi020704g; PMID: 12718522
- Kind J, van Steensel B. Genome-nuclear lamina interactions and gene regulation. Curr Opin Cell Biol 2010; 22:320 - 5; http://dx.doi.org/10.1016/j.ceb.2010.04.002; PMID: 20444586
- Pickersgill H, Kalverda B, de Wit E, Talhout W, Fornerod M, van Steensel B. Characterization of the Drosophila melanogaster genome at the nuclear lamina. Nat Genet 2006; 38:1005 - 14; http://dx.doi.org/10.1038/ng1852; PMID: 16878134
- Guelen L, Pagie L, Brasset E, Meuleman W, Faza MB, Talhout W, Eussen BH, de Klein A, Wessels L, de Laat W, et al. Domain organization of human chromosomes revealed by mapping of nuclear lamina interactions. Nature 2008; 453:948 - 51; http://dx.doi.org/10.1038/nature06947; PMID: 18463634
- Peric-Hupkes D, Meuleman W, Pagie L, Bruggeman SW, Solovei I, Brugman W, Gräf S, Flicek P, Kerkhoven RM, van Lohuizen M, et al. Molecular maps of the reorganization of genome-nuclear lamina interactions during differentiation. Mol Cell 2010; 38:603 - 13; http://dx.doi.org/10.1016/j.molcel.2010.03.016; PMID: 20513434
- van Bemmel JG, Pagie L, Braunschweig U, Brugman W, Meuleman W, Kerkhoven RM, van Steensel B. The insulator protein SU(HW) fine-tunes nuclear lamina interactions of the Drosophila genome. PLoS One 2010; 5:e15013; http://dx.doi.org/10.1371/journal.pone.0015013; PMID: 21124834
- McCord RP, Nazario-Toole A, Zhang H, Chines PS, Zhan Y, Erdos MR, Collins FS, Dekker J, Cao K. Correlated alterations in genome organization, histone methylation, and DNA-lamin A/C interactions in Hutchinson-Gilford progeria syndrome. Genome Res 2013; 23:260 - 9; http://dx.doi.org/10.1101/gr.138032.112; PMID: 23152449
- Kind J, Pagie L, Ortabozkoyun H, Boyle S, de Vries SS, Janssen H, Amendola M, Nolen LD, Bickmore WA, van Steensel B. Single-cell dynamics of genome-nuclear lamina interactions. Cell 2013; 153:178 - 92; http://dx.doi.org/10.1016/j.cell.2013.02.028; PMID: 23523135
- Sadaie M, Salama R, Carroll T, Tomimatsu K, Chandra T, Young AR, Narita M, Pérez-Mancera PA, Bennett DC, Chong H, et al. Redistribution of the Lamin B1 genomic binding profile affects rearrangement of heterochromatic domains and SAHF formation during senescence. Genes Dev 2013; 27:1800 - 8; http://dx.doi.org/10.1101/gad.217281.113; PMID: 23964094
- Shah PP, Donahue G, Otte GL, Capell BC, Nelson DM, Cao K, Aggarwala V, Cruickshanks HA, Rai TS, McBryan T, et al. Lamin B1 depletion in senescent cells triggers large-scale changes in gene expression and the chromatin landscape. Genes Dev 2013; 27:1787 - 99; http://dx.doi.org/10.1101/gad.223834.113; PMID: 23934658
- Meuleman W, Peric-Hupkes D, Kind J, Beaudry JB, Pagie L, Kellis M, Reinders M, Wessels L, van Steensel B. Constitutive nuclear lamina-genome interactions are highly conserved and associated with A/T-rich sequence. Genome Res 2013; 23:270 - 80; http://dx.doi.org/10.1101/gr.141028.112; PMID: 23124521
- Kubben N, Adriaens M, Meuleman W, Voncken JW, van Steensel B, Misteli T. Mapping of lamin A- and progerin-interacting genome regions. Chromosoma 2012; 121:447 - 64; http://dx.doi.org/10.1007/s00412-012-0376-7; PMID: 22610065
- Lund E, Oldenburg AR, Delbarre E, Freberg CT, Duband-Goulet I, Eskeland R, Buendia B, Collas P. Lamin A/C-promoter interactions specify chromatin state-dependent transcription outcomes. Genome Res 2013; 23:1580 - 9; http://dx.doi.org/10.1101/gr.159400.113; PMID: 23861385
- Zuleger N, Robson MI, Schirmer EC. The nuclear envelope as a chromatin organizer. Nucleus 2011; 2:339 - 49; http://dx.doi.org/10.4161/nucl.2.5.17846; PMID: 21970986
- Zuleger N, Boyle S, Kelly DA, de Las Heras JI, Lazou V, Korfali N, Batrakou DG, Randles KN, Morris GE, Harrison DJ, et al. Specific nuclear envelope transmembrane proteins can promote the location of chromosomes to and from the nuclear periphery. Genome Biol 2013; 14:R14; http://dx.doi.org/10.1186/gb-2013-14-2-r14; PMID: 23414781
- Andrés V, González JM. Role of A-type lamins in signaling, transcription, and chromatin organization. J Cell Biol 2009; 187:945 - 57; http://dx.doi.org/10.1083/jcb.200904124; PMID: 20038676
- Dauer WT, Worman HJ. The nuclear envelope as a signaling node in development and disease. Dev Cell 2009; 17:626 - 38; http://dx.doi.org/10.1016/j.devcel.2009.10.016; PMID: 19922868
- Schreiber KH, Kennedy BK. When lamins go bad: nuclear structure and disease. Cell 2013; 152:1365 - 75; http://dx.doi.org/10.1016/j.cell.2013.02.015; PMID: 23498943
- Gundersen GG, Worman HJ. Nuclear positioning. Cell 2013; 152:1376 - 89; http://dx.doi.org/10.1016/j.cell.2013.02.031; PMID: 23498944
- Worman HJ. Nuclear lamins and laminopathies. J Pathol 2012; 226:316 - 25; http://dx.doi.org/10.1002/path.2999; PMID: 21953297
- Mattout A, Pike BL, Towbin BD, Bank EM, Gonzalez-Sandoval A, Stadler MB, Meister P, Gruenbaum Y, Gasser SM. An EDMD mutation in C. elegans lamin blocks muscle-specific gene relocation and compromises muscle integrity. Curr Biol 2011; 21:1603 - 14; http://dx.doi.org/10.1016/j.cub.2011.08.030; PMID: 21962710
- Choi JC, Worman HJ. Reactivation of autophagy ameliorates LMNA cardiomyopathy. Autophagy 2013; 9:110 - 1; http://dx.doi.org/10.4161/auto.22403; PMID: 23044536
- Ho CY, Jaalouk DE, Vartiainen MK, Lammerding J. Lamin A/C and emerin regulate MKL1-SRF activity by modulating actin dynamics. Nature 2013; 497:507 - 11; http://dx.doi.org/10.1038/nature12105; PMID: 23644458
- Reddy KL, Zullo JM, Bertolino E, Singh H. Transcriptional repression mediated by repositioning of genes to the nuclear lamina. Nature 2008; 452:243 - 7; http://dx.doi.org/10.1038/nature06727; PMID: 18272965
- Towbin BD, González-Aguilera C, Sack R, Gaidatzis D, Kalck V, Meister P, Askjaer P, Gasser SM. Step-wise methylation of histone H3K9 positions heterochromatin at the nuclear periphery. Cell 2012; 150:934 - 47; http://dx.doi.org/10.1016/j.cell.2012.06.051; PMID: 22939621
- Zullo JM, Demarco IA, Piqué-Regi R, Gaffney DJ, Epstein CB, Spooner CJ, Luperchio TR, Bernstein BE, Pritchard JK, Reddy KL, et al. DNA sequence-dependent compartmentalization and silencing of chromatin at the nuclear lamina. Cell 2012; 149:1474 - 87; http://dx.doi.org/10.1016/j.cell.2012.04.035; PMID: 22726435
- Boquest AC, Shahdadfar A, Frønsdal K, Sigurjonsson O, Tunheim SH, Collas P, Brinchmann JE. Isolation and transcription profiling of purified uncultured human stromal stem cells: alteration of gene expression after in vitro cell culture. Mol Biol Cell 2005; 16:1131 - 41; http://dx.doi.org/10.1091/mbc.E04-10-0949; PMID: 15635089
- An G, Miner CA, Nixon JC, Kincade PW, Bryant J, Tucker PW, Webb CF. Loss of Bright/ARID3a function promotes developmental plasticity. Stem Cells 2010; 28:1560 - 7; http://dx.doi.org/10.1002/stem.491; PMID: 20680960
- Webb CF, Bryant J, Popowski M, Allred L, Kim D, Harriss J, Schmidt C, Miner CA, Rose K, Cheng HL, et al. The ARID family transcription factor bright is required for both hematopoietic stem cell and B lineage development. Mol Cell Biol 2011; 31:1041 - 53; http://dx.doi.org/10.1128/MCB.01448-10; PMID: 21199920
- Johnson N, Krebs M, Boudreau R, Giorgi G, LeGros M, Larabell C. Actin-filled nuclear invaginations indicate degree of cell de-differentiation. Differentiation 2003; 71:414 - 24; http://dx.doi.org/10.1046/j.1432-0436.2003.7107003.x; PMID: 12969334
- Fricker M, Hollinshead M, White N, Vaux D. Interphase nuclei of many mammalian cell types contain deep, dynamic, tubular membrane-bound invaginations of the nuclear envelope. J Cell Biol 1997; 136:531 - 44; http://dx.doi.org/10.1083/jcb.136.3.531; PMID: 9024685
- Mikkelsen TS, Ku M, Jaffe DB, Issac B, Lieberman E, Giannoukos G, Alvarez P, Brockman W, Kim TK, Koche RP, et al. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature 2007; 448:553 - 60; http://dx.doi.org/10.1038/nature06008; PMID: 17603471
- Polioudaki H, Kourmouli N, Drosou V, Bakou A, Theodoropoulos PA, Singh PB, Giannakouros T, Georgatos SD. Histones H3/H4 form a tight complex with the inner nuclear membrane protein LBR and heterochromatin protein 1. EMBO Rep 2001; 2:920 - 5; http://dx.doi.org/10.1093/embo-reports/kve199; PMID: 11571267
- Somech R, Shaklai S, Geller O, Amariglio N, Simon AJ, Rechavi G, Gal-Yam EN. The nuclear-envelope protein and transcriptional repressor LAP2beta interacts with HDAC3 at the nuclear periphery, and induces histone H4 deacetylation. J Cell Sci 2005; 118:4017 - 25; http://dx.doi.org/10.1242/jcs.02521; PMID: 16129885
- Holaska JM, Lee KK, Kowalski AK, Wilson KL. Transcriptional repressor germ cell-less (GCL) and barrier to autointegration factor (BAF) compete for binding to emerin in vitro. J Biol Chem 2003; 278:6969 - 75; http://dx.doi.org/10.1074/jbc.M208811200; PMID: 12493765
- Foisner R, Gerace L. Integral membrane proteins of the nuclear envelope interact with lamins and chromosomes, and binding is modulated by mitotic phosphorylation. Cell 1993; 73:1267 - 79; http://dx.doi.org/10.1016/0092-8674(93)90355-T; PMID: 8324822
- Lin F, Blake DL, Callebaut I, Skerjanc IS, Holmer L, McBurney MW, Paulin-Levasseur M, Worman HJ. MAN1, an inner nuclear membrane protein that shares the LEM domain with lamina-associated polypeptide 2 and emerin. J Biol Chem 2000; 275:4840 - 7; http://dx.doi.org/10.1074/jbc.275.7.4840; PMID: 10671519
- Ye Q, Worman HJ. Primary structure analysis and lamin B and DNA binding of human LBR, an integral protein of the nuclear envelope inner membrane. J Biol Chem 1994; 269:11306 - 11; PMID: 8157662
- Ikegami K, Egelhofer TA, Strome S, Lieb JD. Caenorhabditis elegans chromosome arms are anchored to the nuclear membrane via discontinuous association with LEM-2. Genome Biol 2010; 11:R120; http://dx.doi.org/10.1186/gb-2010-11-12-r120; PMID: 21176223
- Henikoff S. Nucleosome destabilization in the epigenetic regulation of gene expression. Nat Rev Genet 2008; 9:15 - 26; http://dx.doi.org/10.1038/nrg2206; PMID: 18059368
- Chen H, Chen X, Zheng Y. The nuclear lamina regulates germline stem cell niche organization via modulation of EGFR signaling. Cell Stem Cell 2013; 13:73 - 86; http://dx.doi.org/10.1016/j.stem.2013.05.003; PMID: 23827710
- Collas P, Lund EG, Oldenburg AR. Closing the (nuclear) envelope on the genome. Bioessays 2013; In press
- Mikkelsen TS, Xu Z, Zhang X, Wang L, Gimble JM, Lander ES, Rosen ED. Comparative epigenomic analysis of murine and human adipogenesis. Cell 2010; 143:156 - 69; http://dx.doi.org/10.1016/j.cell.2010.09.006; PMID: 20887899
- Steger DJ, Grant GR, Schupp M, Tomaru T, Lefterova MI, Schug J, Manduchi E, Stoeckert CJ Jr., Lazar MA. Propagation of adipogenic signals through an epigenomic transition state. Genes Dev 2010; 24:1035 - 44; http://dx.doi.org/10.1101/gad.1907110; PMID: 20478996
- Mousavi K, Zare H, Dell’orso S, Grontved L, Gutierrez-Cruz G, Derfoul A, Hager GL, Sartorelli V. eRNAs promote transcription by establishing chromatin accessibility at defined genomic loci. Mol Cell 2013; 51:606 - 17; http://dx.doi.org/10.1016/j.molcel.2013.07.022; PMID: 23993744
- Nagano T, Lubling Y, Stevens TJ, Schoenfelder S, Yaffe E, Dean W, Laue ED, Tanay A, Fraser P. Single-cell Hi-C reveals cell-to-cell variability in chromosome structure. Nature 2013; 502:59 - 64; http://dx.doi.org/10.1038/nature12593; PMID: 24067610