Abstract
Alus are transposable elements belonging to the short interspersed element family. They occupy over 10% of human genome and have been spreading through genomes over the past 65 million years. In the past, they were considered junk DNA with little function that took up genome volumes. Today, Alus and other transposable elements emerge to be key players in cellular function, including genomic activities, gene expression regulations, and evolution. Here we summarize the current understanding of Alu function in genome and gene expression regulation in human cell nuclei.
Introduction
Transposable elements (TE) spread and extend genomes of various species through insertion and amplification. They are believed to play critical roles in the remodeling and controlling of genome function in response to environmental pressures and in speciation.Citation1 Approximately 44% of the human genome is composed of TE (http://genome.ucsc.edu), some of which remain active at an estimated rate of 1 insertion in 10–100 live births.Citation2-Citation4 Over 60 years ago, Barbara McClintockCitation5 discovered transposition and proposed that TE modifies and controls maize gene expression. Not till recently has the breadth of the significance of TEs in genome function come into focus. Increasing numbers of studies have explored various aspects of TE elements. These studies are well reviewed by several groups.Citation1,Citation6-Citation8 This review focuses on the Alu family, which is a member of the small interspersed elements (SINEs), a group of RNA-mediated retrotransposable elements.
Alu occupies approximately 10% of the human genome (~1.1 million copies), making it the largest TE family in human genome.Citation9-Citation11Alus were discovered by their shared sensitivity to digestion by restriction enzyme AluI.Citation12 Derived from 7SL RNA gene, Alu elements have been propagating along with the evolution of primates over the past 65 million years and are primate specific.Citation13,Citation14 Based on their age, Alus are classified into several subfamilies, Alu J, S, and Y, from the oldest to the youngest in that order. The younger variants remain active in transposition. Several published reviews summarize the significance of Alu in primate biology from their role in evolution, in diseases, and in gene expression.Citation8,Citation15-Citation17 This article summarizes the function of Alus in human nuclei.
The Function of Non-Transcribed Genomic Alus
Active Alu transposition and amplification diversify genomes and cause diseases
Alus are believed to have retrotransposed with much higher frequency at earlier stages of primate evolution, with the rate of transposition declining from 1 per live birth then to about 1 in 20 live births today.Citation2,Citation18 Given the current large human population, the number of collective insertions remains to be highly significant. Over time, these insertions, together with recombination, truncations, amplifications, and mutations, allow Alus to assimilate and to co-evolve with the human genome.
Alu transposition is determined by the sequences of the Alus and their ability to interact with SRP9/14 to form unique RNPs. Transposition capable free Alus are transcribed by RNA pol III. As the older Alus continue to change over time due to mutations, losing consensus sequences required for the transposition, the current insertion events are primarily attributed to the mobility of younger Alus.Citation19 It is essential for the transposition capable Alu RNAs to bind SRP9/14 proteins, which form the pre-requisite intermediates prior to the reverse transcription for transpositions.Citation20 The resulting retrotransposition generally create new insertions, most of which are deleterious to genome function.Citation21 Thus, cellular mechanisms are in place to control the expression of mobile Alus. Regulation takes place both at transcription and post-transcriptional levels. At the transcriptional level, most mobile Alus remains silent through epigenetic silencing mechanisms including methylation to CpGs within Alus and histone modifications.Citation22 The transcriptional silencing explains why only very few Alu genes (~150) are transcribed, many of which are not transposition competent.Citation19,Citation23-Citation25 At the post-transcriptional level, MOV10 RNA helicase function is found to restrict transposition of Alus and other TEs through binding and targeting them to stress granules, preventing the replication and thus the insertion of TEs.Citation26
Alu insertions, while can be beneficial, are mostly disruptive, leading to lethality or diseases, accounting for approximately 0.4% of all human diseases.Citation18 Over a long period of time, the slow adaptation of Alus in the genome is believed to be an engine that drives the primate evolution.Citation8 The insertion, recombination, and amplification, leading to the spreading and expansion of Alus and other TEs across the genome help develop diversity and complexity along with the genome evolution,Citation8,Citation15,Citation27-Citation29 which might contribute to the rise of human. For example, Alu mediated RNA editing is shown to be critical for cognitive and behavioral functionCitation30 and defects in RNA editing predisposes people to neurodegenerative diseases.Citation31 As the inverted Alus embedded in pre/mRNA are substrates for editing and the level of editing is substantially increased in human vs. mice, the editing in Alus may have direct roles in the advanced neuronal development in human.Citation32 Additionally, genomic sequencing and mapping among human populations demonstrate that Alus and Alu related polymorphisms contribute greatly to the diversity among humans.
However, Alu insertions and non-allelic homologous recombination between Alus cause chromosome instability or cause disruptions of gene structures and functions, leading to diseases in surviving individuals. For example, neurofibromatosis, a genetic disorder associated with childhood neurological tumors, is caused by changes in neurofibromatosis gene, NF1. Analyses of patient genomes show that insertions of as much as 14 Alus and other TE elements are responsible for the disease causing changes in splicing patterns of the gene, rendering it dysfunctional.Citation33 Through this mechanism, Alus are responsible for a large number of diseases.Citation18 Interestingly, not all genes are equally targeted by Alu insertions and recombination. Some are found to be preferred hot spots for these events, such as LDLR gene for hypercholesterolemia,Citation34-Citation38BRAC1,Citation39,Citation40 and BRCA2Citation41,Citation42 for breast cancer, and so on.
Being a large part of the genome, Alus in cis directly contribute to gene expression regulations
The idea that TEs control and regulate gene expression was first suggested by Barbara McClintockCitation5,Citation43 in the studies of maize genome. Since then, it is increasingly realized that TEs are the binding sites for various transcription factors and regulators.Citation21 In humans, 39.4% of all transcription start sites are located within TEs.Citation44 The large proportion of these sites is derived from ancient inserts that have been adapted through exaptation over a long period time co-evolving with the transcriptional regulators.Citation45 Some of these elements (living fossils) are found conserved in distant species tracing back over 400 million years.Citation46 Alus as a class of newer TEs (65 million years) have also been shown to create many transcription start sites. They are the binding sites for a dozen of transcription factors including SP1, PITX2, LUN1, etc., and many of these sites are associated with early developmental genes.Citation47 More recently methylation and deamination of CpGs (C→G) in Alus are found to create functional binding sites for p53, myc, ANRIL, and PAX-6.Citation48-Citation51 These findings demonstrate that the exaptation of Alus in the genome may allow for higher primate specific regulations of critical gene expressions. It would not be surprising for future analyses to implicate Alus in more transcription factor binding events.
Alus, as with other TEs, are frequently hot spots for recombination and DNA damage
The large number of copies in the genome and the transposable nature of Alu repeats provide enormous opportunities for recombination events that induce non-allelic homologous recombination among chromosomes, resulting alteration of the human genome. These events can take place at various stages of organismal development and at different physiological environments. They can be observed in germ lines, in somatic cells, and in cells under environmental stresses.Citation15 For example, a focused analysis of Alu Y insertions found significant increases in recombination rate within 2 kb of the Alu among several human populations.Citation52 However, not all of the events share the same efficiency.Citation15 The longer of the homolog sequences in a combinatory pair corresponds to more efficient recombination.Citation53 Alu mediated recombination is one of the major cause for genome instability and chromosome translocation, underlining various diseasesCitation54,Citation55 and malignancy.Citation56-Citation58 Closely related to recombination, Alus are (as with other TEs) sites prone to DNA damage. Alus are recently found enriched in chromosome common fragile sites,Citation59 which tend to break, although they are part of normal chromosome structure and play an important function in sister chromatid exchange and other recombinatory functions.Citation60,Citation61 Another recent study shows that the majority of early replicating fragile sites in human cells are sites containing repetitive TEs, Alu being one of them.Citation62 These sites are also sensitive to damage during environmental stresses and under oncogenic pressures.Citation62 Together these observations indicate that Alus (as with other TEs) play important roles in the dynamics of DNA breakage and recombination, which are part of essential genome function during development and tissues specific responses to environmental stresses. However, when they are not well controlled, the induced genome instability serves as basis for diseases, such as malignancy.Citation63
Transcribed Alu
Alus are primarily transcribed by RNA pol II and pol III. The majority of Pol II transcribed Alus are present in introns and the remaining ones are in the untranslated regions of mRNAs. Alus are rarely associated with exons. Increasingly, pre-mRNA associated Alus are found to play important roles in regulating gene expression at pre/mRNA levels. Pol III transcribes free or core unembedded Alu of about 280 bps. Much less is known regarding the function of these Alus. Additional to be the precursor of retrotransposon, they also play critical roles in cellular functions.Citation21
Alus embedded in pre/mRNA
Exonization mediated by Alus in introns can significantly influence the function of the translated proteins. Exonization is a process, in which a normally non-exon element in the intron is spliced into an exon of mRNA either in cis (within the same transcript) or in trans (between different transcripts).Citation64 Alu is present in over 50% of introns and consensus Alu contains 23 potential splice sites, 19 of which are in the minor strand, consistent with the observation that 85% Alu exons come from antisense Alu elements.Citation65 The abundance of Alu in introns makes it a leading force of new exon formation, contributing to 64% of new exons.Citation66 Although 585 Alu exons were annotated previously, an additional 1318 cryptic exons originated from Alus have recently been identified.Citation67
Alu-mediated exonization takes place primarily through two mechanisms. The potential or cryptic splice sites in the Alu concensus sequences form pseudosplice sites. Specific mutations possibly over multiple steps turn Alu pseudosplice sites into bonafide sites.Citation68,Citation69 In analyses of 13 primate individuals, Singer et al., mapped out the stepwise mutagenesis over millions of years that generated an alternative 5′ exon in the human tumor necrosis factor receptor gene.Citation70 Additionally, insertion of inverted Alu allows for a stretch of Us incorporating into pre-mRNA, together with desirable mutations, making it into a functional splice site for exon generation. Therefore, mutations over time contribute to the conversion of pseudosplice sites within Alus into functional ones. A-I editing of Alu containing RNA is another mechanism that facilitates Alu exonization.Citation64,Citation71,Citation72 Lev-Maor et al. found that the second A of the AA upstream of exon 8 of nuclear prelamin recognition factor is edited from A-I, effectively turning AA into AG and making it a splice site. The editing is mediated through the formation of a double strand RNA stem loop of this Alu with an inverted Alu 30 bp upstream. Incidentally, the new exon also contains TAG, a premature stop codon that is corrected also by editing A into G, forming harmless TGG. The newly formed exon is alternatively spliced in a tissue-specific manner.Citation72 This is an intriguing example where a double editing event together with an alternative splicing prevents the new Alu exon from being lethal in the final product, thus allowing the survival of the new exon. However, Alu mediated aberrant splicing is much more often disruptive of gene function, leading to diseases. A number of hereditary diseases are the results of Alu induced cryptic exon splicing, including Hermansky-Pudlak syndrome, Ataxia-telangiectasia, Afibrinogenemia, and chronic granulomatous disease, etc.Citation73
Because of the detrimental impacts of most exonizations, stringent mechanisms are in place to recognize only the intended exons. Most exonization is thus accompanied with alternative splicing, which excludes the use of the potentially harmful exons in specific tissues. The finding that new exons are more often alternatively spliced than older ones supports the role of this mechanism in adapting new exons.Citation65,Citation69,Citation74 An alternative protection mechanism is to restrict access to the Alu cryptical splice sites by splicing factors.Citation67 For general splicing, U2AF65 binds exonal signals to initiate the recruitment of splicosomes.Citation75 A recent study shows that hnRNP C competes with U2AF65 for the binding of cryptical exonal signals through interacting with a stretch of Us.Citation67 In the absence of hnRNPC, U2AF65 binding to Alu exonal signal significantly increases, directly corresponding to the significant increases of Alu mediated exonization globally. The results demonstrate that hnRNP C plays a key role in minimizing Alu exon inclusion under normal circumstance by blocking the access of U2AF65 to cryptic splice sites.Citation67
Over time, the adapted Alu intiated exonization survivesCitation76 and directly contributes to the complexity of human genome function. For example, ADAR, an RNA editing enzyme, has 4 alternative spliced forms in human cells. One of which derives from the inclusion of an Alu-initiated exonization, leading to 40 extra amino acids in the protein. This inclusion has since been adapted as a part of isoforms of the deaminase and is highly expressed in neuronal tissues.Citation76 Mattick and MehlerCitation32 proposed that the adaptation of Alus through selection could contribute to the higher order of cognitive function in humans. The Alus could be the critical element that sets human apart from other primates.Citation8,Citation27
Alu at the 3′ UTR is a critical regulatory element for mRNA metabolism. While majority of the intragenic Alus are in the introns, approximately 5% of mRNA contains Alus and 82% of them locate at the 3′ UTR.Citation77Alu elements at 3′UTR have been shown to regulate mRNA functions through several mechanisms, including RNA editing, nuclear retention, polyadenylation, RNA stability, miRNA function, and translational regulation in the cytoplasm. As this review focuses on the Alu function in the nucleus, we will discuss mostly their roles in the nuclear retention of mRNA, editing, and polyadenylation.
Alus at the 3′ UTR are found to regulate the nuclear retention of mRNA. mCAT2, a cation transporter protein, encodes two isoforms through the use of two promoters. The short form is transported into the cytoplasm and is translated at steady-state. The longer form of the mRNA is predominantly retained in the nucleus.Citation78 It is only released into cytoplasm under stresses, such as viral infection and IFN gamma responses. The nuclear retained RNA is primarily enriched in the paraspeckles. The 3′ UTR contains 3 reverse and 1 forward Alu elements. Their presence is essential for nuclear retention.Citation78 The forward repetitive element is a substrate for editing and is hyper-edited. It is believed that the editing of these repetitive elements helps explain the nuclear retention of these RNA. Subsequently, Chen et al. find that inverted Alu at 3′UTR is responsible for editing and nuclear retention using ectopically expressed reporter constructs.Citation79 However, it is not entirely clear whether editing alone can explains the nuclear retention of the inverted Alu containing mRNA. Two more recent studies show that an endogenous mRNA or a reporter mRNA with edited inverted Alus at the 3′ UTR exports to the cytoplasm rather than being retained in the nucleus.Citation80,Citation81 These findings suggest that editing may not be the key to nuclear retention of at least some mRNA with inverted Alu in 3′ UTR. In ES cells without detectable paraspeckles, mRNAs with extensive editing at the 3′ UTR with inverted Alu, are effectively transported into the cytoplasm.Citation82 A recent study begins to shed light regarding the export of mRNA with inverted Alus containing 3′UTR. Elbarbary et al.Citation83 demonstrate that binding of inverted Alu at 3′ UTR with STAU1 proteins (dsRNA-binding protein Staufen1) inhibits the nuclear retention of these RNA, thus promoting the export of the mRNA.Citation83 These findings together suggest that the nuclear retention of mRNA with Alu at 3′ UTR could be the results of the regulated balance between exporting forces (binding to STAU1, etc.) and retention power (editing, and other yet to be revealed functions).
Reversed Alus with two stretches of A rich sequences embedded in coding genes can also be potential polyadenylation signals. A single point mutation turning As to AATAAA, a conserved polyadenylation sites, can induce premature termination of transcription and generates truncated proteins. While this can be harmful, it is also a way to generate tolerated diversity.Citation17 Analyses of human genome indicates that some of the genes use Alus as polyadenylation sitesCitation84,Citation85 and among them, some of which distinguish human transcripts from other primates,Citation86 suggesting a role of these adapted Alu polyadenylation sites in human evolution.
Alus at the 3′ UTR can also serve as targets for miRNA regulations. There is a significant number of miRNAs with sequences that are complementary to Alus.Citation87-Citation89 A primate specific gene cluster in chromosome 19 is found to encode miRNAs that target the most conserved part of sense Alus.Citation15 The majority of the 3′UTR-located Alus carries potential target sites for at least 53 miRNAs.Citation87,Citation89
Transcribed free Alu by pol III
Although there are more than a million copies of Alu spread in the human genome, very few are transcribed independently. Most of transcribed Alus are those embedded in pre-mRNA and transcribed by RNA polymerase II. RNA pol III is responsible for transcribing free Alu (~280 bps), which contains an internal promoter for class two pol III transcription.Citation19,Citation23 As Alus are ubiquitous throughout coding sequence, RT-PCR is not representative of free Alu transcription. Over the past few years, the genome-wide ChIP for pol III transcription machinery from three groups mapped the active Alu transcribing loci in genomes of several human cell lines,Citation19,Citation23-Citation25 revealing for the first time the specific Alus that are transcribed. More than 150 loci are found to be active in various cells. While many loci are overlapping among different cell lines, some are unique to specific cell lines,Citation19 The majority of pol III associated loci are those of older families including Alu S and Alu J and most of these transcripts lack sequences required for retrotransposition, suggesting that these are not the main source of active retrotransposition.Citation19
What then is the function of the non-transposible Alu RNA? Heat shock and translation inhibitors have been shown to activate the transcription of free Alus.Citation90 The similar elevation was also found during viral infection.Citation91,Citation92 The transcription enhancement is highly regulated and the levels of Alu RNA return to the basal level upon relief from the stresses. The increases in the free Alus RNA upon stresses directly correspond to the inhibition of RNA pol II transcription in the nucleus.Citation93 The mechanistic studies show that Alu RNA directly binds RNA pol II as tested both in vitro and in vivo, suggesting that Alu RNA integrates into the transcriptional complex, disrupting the interaction between pol II and promoter DNA and blocking transcriptional initiation.Citation94 Additionally, elevated levels of free Alus may contribute to cellular senescence.Citation95 Transcription of Alu in reverse orientation can also act as a cis natural antisense transcripts (NAT).Citation96 The NATs regulate gene expression at both transcriptional and post-transcriptional levels. Transcription at opposite orientations on the same DNA template slows down progression of polymerases possibly to avoid collision.Citation97 At the post-transcriptional levels, NATs can act through antisense RNA or small RNA interference mechanisms.Citation98 Recently, studies of a retinoic acid responsive and pol III transcribed DR2 Alus show that the small RNAs derived from dicer processed DR2 Alus play critical role in the degradation of stem cell specific RNAs during cellular differentiation.Citation99
Cytoplasmic free Alu RNAs have multi-facet functions. Alus are found to regulate translation of mRNA, in which Alu RNAs form RNP with SRP protein, SRP9/14. The resulting Alu RNPs inhibit double strand RNA-dependent protein kinase (PKR), leading to translational stimulation.Citation64,Citation100-Citation103 The free Alus RNAs are found to help prevent miRNA targeting to targets that are within Alus at the 3′ UTR, possibly by blocking access to the targets, thus reducing miRNA regulatory function to the specific RNAs.Citation104 Furthermore, free Alus pairing with 3′UTR Alus in trans can activate mRNA decay.Citation105,Citation106 These findings demonstrate that Alus have multiple and unique functions in human cells. As the human genome contains a large number of Alu genes, we will probably see more cellular functions to be assigned to Alu RNAs and perhaps they are critical in making us human.
Summary
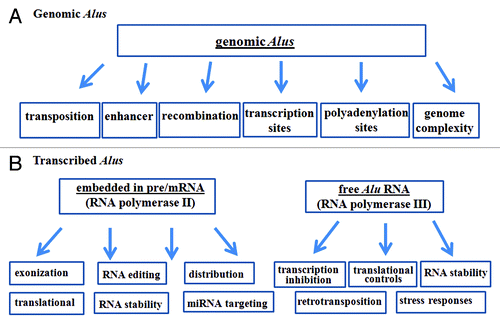
Alu, although relatively young in the evolution history (65 million years), is the most successful transposable element in the human genome, occupying over 10% of the genome. They function in two forms, as genomic elements or as transcribed RNA (). Alu-mediated transposition, DNA damage, mutations, and recombination directly contribute to the complexity and instability of the genome. Alus, as with other TEs play key roles in gene expression at the transcriptional level. They are frequently the transcription start sites and transcription factor binding sites. These sites appear to co-evolve with transcription and replication machineries over time to allow for specific regulations, which could be critical for speciation. When embedded in pre/mRNA, Alus regulate the diversity of mRNA through editing and exonization and influence the stability and translatability of the mRNAs. Furthermore, free Alu RNAs regulate genome functions from pol II transcription and retrotransposition to antisense regulation of gene expression under normal and stressed conditions. Altogether, these highly diverse functions of Alus mediate genetic drift, increase complexity of the genome function, and are likely to play critical roles in human evolution.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Acknowledgments
We are grateful to the critical read of Dr Barry Feldman, and the work is supported by grants from NIH (2R01GM078555–05).
References
- Rebollo R, Horard B, Hubert B, Vieira C. Jumping genes and epigenetics: Towards new species. Gene 2010; 454:1 - 7; http://dx.doi.org/10.1016/j.gene.2010.01.003; PMID: 20102733
- Cordaux R, Hedges DJ, Herke SW, Batzer MA. Estimating the retrotransposition rate of human Alu elements. Gene 2006; 373:134 - 7; http://dx.doi.org/10.1016/j.gene.2006.01.019; PMID: 16522357
- Kazazian HH Jr.. An estimated frequency of endogenous insertional mutations in humans. Nat Genet 1999; 22:130; http://dx.doi.org/10.1038/9638; PMID: 10369250
- Li X, Scaringe WA, Hill KA, Roberts S, Mengos A, Careri D, Pinto MT, Kasper CK, Sommer SS. Frequency of recent retrotransposition events in the human factor IX gene. Hum Mutat 2001; 17:511 - 9; http://dx.doi.org/10.1002/humu.1134; PMID: 11385709
- McCLINTOCK B. Chromosome organization and genic expression. Cold Spring Harb Symp Quant Biol 1951; 16:13 - 47; http://dx.doi.org/10.1101/SQB.1951.016.01.004; PMID: 14942727
- Batzer MA, Schmid CW, Deininger PL. Evolutionary analyses of repetitive DNA sequences. Methods Enzymol 1993; 224:213 - 32; http://dx.doi.org/10.1016/0076-6879(93)24017-O; PMID: 8264388
- Ponicsan SL, Kugel JF, Goodrich JA. Genomic gems: SINE RNAs regulate mRNA production. Curr Opin Genet Dev 2010; 20:149 - 55; http://dx.doi.org/10.1016/j.gde.2010.01.004; PMID: 20176473
- Schmitz J. SINEs as driving forces in genome evolution. Genome Dyn 2012; 7:92 - 107; http://dx.doi.org/10.1159/000337117; PMID: 22759815
- Rubin CM, Houck CM, Deininger PL, Friedmann T, Schmid CW. Partial nucleotide sequence of the 300-nucleotide interspersed repeated human DNA sequences. Nature 1980; 284:372 - 4; http://dx.doi.org/10.1038/284372a0; PMID: 6244506
- Jelinek WR, Toomey TP, Leinwand L, Duncan CH, Biro PA, Choudary PV, Weissman SM, Rubin CM, Houck CM, Deininger PL, et al. Ubiquitous, interspersed repeated sequences in mammalian genomes. Proc Natl Acad Sci U S A 1980; 77:1398 - 402; http://dx.doi.org/10.1073/pnas.77.3.1398; PMID: 6246492
- Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, et al, International Human Genome Sequencing Consortium. Initial sequencing and analysis of the human genome. Nature 2001; 409:860 - 921; http://dx.doi.org/10.1038/35057062; PMID: 11237011
- Houck CM, Rinehart FP, Schmid CW. A ubiquitous family of repeated DNA sequences in the human genome. J Mol Biol 1979; 132:289 - 306; http://dx.doi.org/10.1016/0022-2836(79)90261-4; PMID: 533893
- Batzer MA, Schmid CW, Deininger PL. Evolutionary analyses of repetitive DNA sequences. Methods Enzymol 1993; 224:213 - 32; http://dx.doi.org/10.1016/0076-6879(93)24017-O; PMID: 8264388
- Ullu E, Tschudi C. Alu sequences are processed 7SL RNA genes. Nature 1984; 312:171 - 2; http://dx.doi.org/10.1038/312171a0; PMID: 6209580
- Batzer MA, Deininger PL. Alu repeats and human genomic diversity. Nat Rev Genet 2002; 3:370 - 9; http://dx.doi.org/10.1038/nrg798; PMID: 11988762
- Hancks DC, Kazazian HH Jr.. Active human retrotransposons: variation and disease. Curr Opin Genet Dev 2012; 22:191 - 203; http://dx.doi.org/10.1016/j.gde.2012.02.006; PMID: 22406018
- Deininger P. Alu elements: know the SINEs. Genome Biol 2011; 12:236; http://dx.doi.org/10.1186/gb-2011-12-12-236; PMID: 22204421
- Deininger PL, Batzer MA. Alu repeats and human disease. Mol Genet Metab 1999; 67:183 - 93; http://dx.doi.org/10.1006/mgme.1999.2864; PMID: 10381326
- Oler AJ, Traina-Dorge S, Derbes RS, Canella D, Cairns BR, Roy-Engel AM. Alu expression in human cell lines and their retrotranspositional potential. Mob DNA 2012; 3:11; http://dx.doi.org/10.1186/1759-8753-3-11; PMID: 22716230
- Bennett EA, Keller H, Mills RE, Schmidt S, Moran JV, Weichenrieder O, Devine SE. Active Alu retrotransposons in the human genome. Genome Res 2008; 18:1875 - 83; http://dx.doi.org/10.1101/gr.081737.108; PMID: 18836035
- Rebollo R, Romanish MT, Mager DL. Transposable elements: an abundant and natural source of regulatory sequences for host genes. Annu Rev Genet 2012; 46:21 - 42; http://dx.doi.org/10.1146/annurev-genet-110711-155621; PMID: 22905872
- Huda A, Mariño-Ramírez L, Jordan IK. Epigenetic histone modifications of human transposable elements: genome defense versus exaptation. Mob DNA 2010; 1:2; http://dx.doi.org/10.1186/1759-8753-1-2; PMID: 20226072
- Canella D, Praz V, Reina JH, Cousin P, Hernandez N. Defining the RNA polymerase III transcriptome: Genome-wide localization of the RNA polymerase III transcription machinery in human cells. Genome Res 2010; 20:710 - 21; http://dx.doi.org/10.1101/gr.101337.109; PMID: 20413673
- Moqtaderi Z, Wang J, Raha D, White RJ, Snyder M, Weng Z, Struhl K. Genomic binding profiles of functionally distinct RNA polymerase III transcription complexes in human cells. Nat Struct Mol Biol 2010; 17:635 - 40; http://dx.doi.org/10.1038/nsmb.1794; PMID: 20418883
- Oler AJ, Alla RK, Roberts DN, Wong A, Hollenhorst PC, Chandler KJ, Cassiday PA, Nelson CA, Hagedorn CH, Graves BJ, et al. Human RNA polymerase III transcriptomes and relationships to Pol II promoter chromatin and enhancer-binding factors. Nat Struct Mol Biol 2010; 17:620 - 8; http://dx.doi.org/10.1038/nsmb.1801; PMID: 20418882
- Goodier JL, Cheung LE, Kazazian HH Jr.. MOV10 RNA helicase is a potent inhibitor of retrotransposition in cells. PLoS Genet 2012; 8:e1002941; http://dx.doi.org/10.1371/journal.pgen.1002941; PMID: 23093941
- Britten RJ. Transposable element insertions have strongly affected human evolution. Proc Natl Acad Sci U S A 2010; 107:19945 - 8; http://dx.doi.org/10.1073/pnas.1014330107; PMID: 21041622
- Iskow RC, McCabe MT, Mills RE, Torene S, Pittard WS, Neuwald AF, Van Meir EG, Vertino PM, Devine SE. Natural mutagenesis of human genomes by endogenous retrotransposons. Cell 2010; 141:1253 - 61; http://dx.doi.org/10.1016/j.cell.2010.05.020; PMID: 20603005
- Huang CR, Schneider AM, Lu Y, Niranjan T, Shen P, Robinson MA, Steranka JP, Valle D, Civin CI, Wang T, et al. Mobile interspersed repeats are major structural variants in the human genome. Cell 2010; 141:1171 - 82; http://dx.doi.org/10.1016/j.cell.2010.05.026; PMID: 20602999
- Valente L, Nishikura K. ADAR gene family and A-to-I RNA editing: diverse roles in posttranscriptional gene regulation. Prog Nucleic Acid Res Mol Biol 2005; 79:299 - 338; http://dx.doi.org/10.1016/S0079-6603(04)79006-6; PMID: 16096031
- Jepson JEC, Reenan RA. Adenosine-to-inosine genetic recoding is required in the adult stage nervous system for coordinated behavior in Drosophila. J Biol Chem 2009; 284:31391 - 400; http://dx.doi.org/10.1074/jbc.M109.035048; PMID: 19759011
- Mattick JS, Mehler MF. RNA editing, DNA recoding and the evolution of human cognition. Trends Neurosci 2008; 31:227 - 33; http://dx.doi.org/10.1016/j.tins.2008.02.003; PMID: 18395806
- Wimmer K, Callens T, Wernstedt A, Messiaen L. The NF1 gene contains hotspots for L1 endonuclease-dependent de novo insertion. PLoS Genet 2011; 7:e1002371; http://dx.doi.org/10.1371/journal.pgen.1002371; PMID: 22125493
- Chae JJ, Park YB, Kim SH, Hong SS, Song GJ, Han KH, Namkoong Y, Kim HS, Lee CC. Two partial deletion mutations involving the same Alu sequence within intron 8 of the LDL receptor gene in Korean patients with familial hypercholesterolemia. Hum Genet 1997; 99:155 - 63; http://dx.doi.org/10.1007/s004390050331; PMID: 9048913
- Lehrman MA, Goldstein JL, Russell DW, Brown MS. Duplication of seven exons in LDL receptor gene caused by Alu-Alu recombination in a subject with familial hypercholesterolemia. Cell 1987; 48:827 - 35; http://dx.doi.org/10.1016/0092-8674(87)90079-1; PMID: 3815525
- Lehrman MA, Schneider WJ, Südhof TC, Brown MS, Goldstein JL, Russell DW. Mutation in LDL receptor: Alu-Alu recombination deletes exons encoding transmembrane and cytoplasmic domains. Science 1985; 227:140 - 6; http://dx.doi.org/10.1126/science.3155573; PMID: 3155573
- Rüdiger NS, Heinsvig EM, Hansen FA, Faergeman O, Bolund L, Gregersen N. DNA deletions in the low density lipoprotein (LDL) receptor gene in Danish families with familial hypercholesterolemia. Clin Genet 1991; 39:451 - 62; http://dx.doi.org/10.1111/j.1399-0004.1991.tb03057.x; PMID: 1863993
- Yamakawa K, Takada K, Yanagi H, Tsuchiya S, Kawai K, Nakagawa S, Kajiyama G, Hamaguchi H. Three novel partial deletions of the low-density lipoprotein (LDL) receptor gene in familial hypercholesterolemia. Hum Genet 1989; 82:317 - 21; http://dx.doi.org/10.1007/BF00273989; PMID: 2544509
- Puget N, Sinilnikova OM, Stoppa-Lyonnet D, Audoynaud C, Pagès S, Lynch HT, Goldgar D, Lenoir GM, Mazoyer S. An Alu-mediated 6-kb duplication in the BRCA1 gene: a new founder mutation?. Am J Hum Genet 1999; 64:300 - 2; http://dx.doi.org/10.1086/302211; PMID: 9915971
- Swensen J, Hoffman M, Skolnick MH, Neuhausen SL. Identification of a 14 kb deletion involving the promoter region of BRCA1 in a breast cancer family. Hum Mol Genet 1997; 6:1513 - 7; http://dx.doi.org/10.1093/hmg/6.9.1513; PMID: 9285788
- Miki Y, Katagiri T, Kasumi F, Yoshimoto T, Nakamura Y. Mutation analysis in the BRCA2 gene in primary breast cancers. Nat Genet 1996; 13:245 - 7; http://dx.doi.org/10.1038/ng0696-245; PMID: 8640237
- Teugels E, De Brakeleer S, Goelen G, Lissens W, Sermijn E, De Grève J. De novo Alu element insertions targeted to a sequence common to the BRCA1 and BRCA2 genes. Hum Mutat 2005; 26:284 - 284; http://dx.doi.org/10.1002/humu.9366; PMID: 16088935
- McClintock B. The significance of responses of the genome to challenge. Science 1984; 226:792 - 801; http://dx.doi.org/10.1126/science.15739260; PMID: 15739260
- Faulkner GJ, Kimura Y, Daub CO, Wani S, Plessy C, Irvine KM, Schroder K, Cloonan N, Steptoe AL, Lassmann T, et al. The regulated retrotransposon transcriptome of mammalian cells. Nat Genet 2009; 41:563 - 71; http://dx.doi.org/10.1038/ng.368; PMID: 19377475
- Feschotte C, Pritham EJ. DNA transposons and the evolution of eukaryotic genomes. Annu Rev Genet 2007; 41:331 - 68; http://dx.doi.org/10.1146/annurev.genet.40.110405.090448; PMID: 18076328
- Bejerano G, Lowe CB, Ahituv N, King B, Siepel A, Salama SR, Rubin EM, Kent WJ, Haussler D. A distal enhancer and an ultraconserved exon are derived from a novel retroposon. Nature 2006; 441:87 - 90; http://dx.doi.org/10.1038/nature04696; PMID: 16625209
- Polak P, Domany E. Alu elements contain many binding sites for transcription factors and may play a role in regulation of developmental processes. BMC Genomics 2006; 7:133; http://dx.doi.org/10.1186/1471-2164-7-133; PMID: 16740159
- Holdt LM, Hoffmann S, Sass K, Langenberger D, Scholz M, Krohn K, Finstermeier K, Stahringer A, Wilfert W, Beutner F, et al. Alu elements in ANRIL non-coding RNA at chromosome 9p21 modulate atherogenic cell functions through trans-regulation of gene networks. PLoS Genet 2013; 9:e1003588; http://dx.doi.org/10.1371/journal.pgen.1003588; PMID: 23861667
- Zemojtel T, Kielbasa SM, Arndt PF, Behrens S, Bourque G, Vingron M. CpG deamination creates transcription factor-binding sites with high efficiency. Genome Biol Evol 2011; 3:1304 - 11; http://dx.doi.org/10.1093/gbe/evr107; PMID: 22016335
- Zemojtel T, Kielbasa SM, Arndt PF, Chung HR, Vingron M. Methylation and deamination of CpGs generate p53-binding sites on a genomic scale. Trends Genet 2009; 25:63 - 6; http://dx.doi.org/10.1016/j.tig.2008.11.005; PMID: 19101055
- Cui F, Sirotin MV, Zhurkin VB. Impact of Alu repeats on the evolution of human p53 binding sites. Biol Direct 2011; 6:2; http://dx.doi.org/10.1186/1745-6150-6-2; PMID: 21208455
- Witherspoon DJ, Watkins WS, Zhang Y, Xing J, Tolpinrud WL, Hedges DJ, Batzer MA, Jorde LB. Alu repeats increase local recombination rates. BMC Genomics 2009; 10:530; http://dx.doi.org/10.1186/1471-2164-10-530; PMID: 19917129
- Waldman AS, Liskay RM. Dependence of intrachromosomal recombination in mammalian cells on uninterrupted homology. Mol Cell Biol 1988; 8:5350 - 7; PMID: 2854196
- Pangrazio A, Caldana ME, Sobacchi C, Panaroni C, Susani L, Mihci E, Cavaliere ML, Giliani S, Villa A, Frattini A. Characterization of a novel Alu-Alu recombination-mediated genomic deletion in the TCIRG1 gene in five osteopetrotic patients. J Bone Miner Res 2009; 24:162 - 7; http://dx.doi.org/10.1359/jbmr.080818; PMID: 18715141
- Purandare SM, Patel PI. Recombination hot spots and human disease. Genome Res 1997; 7:773 - 86; PMID: 9267802
- Lehrman MA, Goldstein JL, Russell DW, Brown MS. Duplication of seven exons in LDL receptor gene caused by Alu-Alu recombination in a subject with familial hypercholesterolemia. Cell 1987; 48:827 - 35; http://dx.doi.org/10.1016/0092-8674(87)90079-1; PMID: 3815525
- Nyström-Lahti M, Kristo P, Nicolaides NC, Chang S-Y, Aaltonen LA, Moisio A-L, Järvinen HJ, Mecklin J-P, Kinzler KW, Vogelstein B, et al. Founding mutations and Alu-mediated recombination in hereditary colon cancer. Nat Med 1995; 1:1203 - 6; http://dx.doi.org/10.1038/nm1195-1203; PMID: 7584997
- Franke G, Bausch B, Hoffmann MM, Cybulla M, Wilhelm C, Kohlhase J, Scherer G, Neumann HPH. Alu-Alu recombination underlies the vast majority of large VHL germline deletions: Molecular characterization and genotype-phenotype correlations in VHL patients. Hum Mutat 2009; 30:776 - 86; http://dx.doi.org/10.1002/humu.20948; PMID: 19280651
- Fungtammasan A, Walsh E, Chiaromonte F, Eckert KA, Makova KD. A genome-wide analysis of common fragile sites: what features determine chromosomal instability in the human genome?. Genome Res 2012; 22:993 - 1005; http://dx.doi.org/10.1101/gr.134395.111; PMID: 22456607
- Durkin SG, Glover TW. Chromosome fragile sites. Annu Rev Genet 2007; 41:169 - 92; http://dx.doi.org/10.1146/annurev.genet.41.042007.165900; PMID: 17608616
- Glover TW, Stein CK. Induction of sister chromatid exchanges at common fragile sites. Am J Hum Genet 1987; 41:882 - 90; PMID: 3674017
- Barlow JH, Faryabi RB, Callén E, Wong N, Malhowski A, Chen HT, Gutierrez-Cruz G, Sun HW, McKinnon P, Wright G, et al. Identification of early replicating fragile sites that contribute to genome instability. Cell 2013; 152:620 - 32; http://dx.doi.org/10.1016/j.cell.2013.01.006; PMID: 23352430
- Hancks DC, Kazazian HH Jr.. Active human retrotransposons: variation and disease. Curr Opin Genet Dev 2012; 22:191 - 203; http://dx.doi.org/10.1016/j.gde.2012.02.006; PMID: 22406018
- Häsler J, Strub K. Alu elements as regulators of gene expression. Nucleic Acids Res 2006; 34:5491 - 7; http://dx.doi.org/10.1093/nar/gkl706; PMID: 17020921
- Sorek R, Ast G, Graur D. Alu-containing exons are alternatively spliced. Genome Res 2002; 12:1060 - 7; http://dx.doi.org/10.1101/gr.229302; PMID: 12097342
- Zhang XH, Chasin LA. Comparison of multiple vertebrate genomes reveals the birth and evolution of human exons. Proc Natl Acad Sci U S A 2006; 103:13427 - 32; http://dx.doi.org/10.1073/pnas.0603042103; PMID: 16938881
- Zarnack K, König J, Tajnik M, Martincorena I, Eustermann S, Stévant I, Reyes A, Anders S, Luscombe NM, Ule J. Direct competition between hnRNP C and U2AF65 protects the transcriptome from the exonization of Alu elements. Cell 2013; 152:453 - 66; http://dx.doi.org/10.1016/j.cell.2012.12.023; PMID: 23374342
- Lev-Maor G, Sorek R, Shomron N, Ast G. The birth of an alternatively spliced exon: 3′ splice-site selection in Alu exons. Science 2003; 300:1288 - 91; http://dx.doi.org/10.1126/science.1082588; PMID: 12764196
- Sorek R, Lev-Maor G, Reznik M, Dagan T, Belinky F, Graur D, Ast G. Minimal conditions for exonization of intronic sequences: 5′ splice site formation in alu exons. Mol Cell 2004; 14:221 - 31; http://dx.doi.org/10.1016/S1097-2765(04)00181-9; PMID: 15099521
- Singer SS, Männel DN, Hehlgans T, Brosius J, Schmitz J. From “junk” to gene: curriculum vitae of a primate receptor isoform gene. J Mol Biol 2004; 341:883 - 6; http://dx.doi.org/10.1016/j.jmb.2004.06.070; PMID: 15328599
- Häsler J, Samuelsson T, Strub K. Useful ‘junk’: Alu RNAs in the human transcriptome. Cell Mol Life Sci 2007; 64:1793 - 800; http://dx.doi.org/10.1007/s00018-007-7084-0; PMID: 17514354
- Lev-Maor G, Sorek R, Levanon EY, Paz N, Eisenberg E, Ast G. RNA-editing-mediated exon evolution. Genome Biol 2007; 8:R29; http://dx.doi.org/10.1186/gb-2007-8-2-r29; PMID: 17326827
- Vorechovsky I. Transposable elements in disease-associated cryptic exons. Hum Genet 2010; 127:135 - 54; http://dx.doi.org/10.1007/s00439-009-0752-4; PMID: 19823873
- Sorek R. The birth of new exons: mechanisms and evolutionary consequences. RNA 2007; 13:1603 - 8; http://dx.doi.org/10.1261/rna.682507; PMID: 17709368
- Warf MB, Diegel JV, von Hippel PH, Berglund JA. The protein factors MBNL1 and U2AF65 bind alternative RNA structures to regulate splicing. Proc Natl Acad Sci U S A 2009; 106:9203 - 8; http://dx.doi.org/10.1073/pnas.0900342106; PMID: 19470458
- Lin L, Shen S, Tye A, Cai JJ, Jiang P, Davidson BL, Xing Y. Diverse splicing patterns of exonized Alu elements in human tissues. PLoS Genet 2008; 4:e1000225; http://dx.doi.org/10.1371/journal.pgen.1000225; PMID: 18841251
- Yulug IG, Yulug A, Fisher EM. The frequency and position of Alu repeats in cDNAs, as determined by database searching. Genomics 1995; 27:544 - 8; http://dx.doi.org/10.1006/geno.1995.1090; PMID: 7558040
- Prasanth KV, Prasanth SG, Xuan Z, Hearn S, Freier SM, Bennett CF, Zhang MQ, Spector DL. Regulating gene expression through RNA nuclear retention. Cell 2005; 123:249 - 63; http://dx.doi.org/10.1016/j.cell.2005.08.033; PMID: 16239143
- Chen L-L, Carmichael GG. Gene regulation by SINES and inosines: biological consequences of A-to-I editing of Alu element inverted repeats. Cell Cycle 2008; 7:3294 - 301; http://dx.doi.org/10.4161/cc.7.21.6927; PMID: 18948735
- Capshew CR, Dusenbury KL, Hundley HA. Inverted Alu dsRNA structures do not affect localization but can alter translation efficiency of human mRNAs independent of RNA editing. Nucleic Acids Res 2012; 40:8637 - 45; http://dx.doi.org/10.1093/nar/gks590; PMID: 22735697
- Fitzpatrick T, Huang S. 3′-UTR-located inverted Alu repeats facilitate mRNA translational repression and stress granule accumulation. Nucleus 2012; 3:359 - 69; http://dx.doi.org/10.4161/nucl.20827; PMID: 22688648
- Chen L-L, Carmichael GG. Altered nuclear retention of mRNAs containing inverted repeats in human embryonic stem cells: functional role of a nuclear noncoding RNA. Mol Cell 2009; 35:467 - 78; http://dx.doi.org/10.1016/j.molcel.2009.06.027; PMID: 19716791
- Elbarbary RA, Li W, Tian B, Maquat LE. STAU1 binding 3′ UTR IRAlus complements nuclear retention to protect cells from PKR-mediated translational shutdown. Genes Dev 2013; 27:1495 - 510; http://dx.doi.org/10.1101/gad.220962.113; PMID: 23824540
- Chen C, Ara T, Gautheret D. Using Alu elements as polyadenylation sites: A case of retroposon exaptation. Mol Biol Evol 2009; 26:327 - 34; http://dx.doi.org/10.1093/molbev/msn249; PMID: 18984903
- Lee JY, Ji Z, Tian B. Phylogenetic analysis of mRNA polyadenylation sites reveals a role of transposable elements in evolution of the 3′-end of genes. Nucleic Acids Res 2008; 36:5581 - 90; http://dx.doi.org/10.1093/nar/gkn540; PMID: 18757892
- Kim DS, Hahn Y. Identification of human-specific transcript variants induced by DNA insertions in the human genome. Bioinformatics 2011; 27:14 - 21; http://dx.doi.org/10.1093/bioinformatics/btq612; PMID: 21037245
- Daskalova E, Baev V, Rusinov V, Minkov I. 3’UTR-located ALU elements: donors of potential miRNA target sites and mediators of network miRNA-based regulatory interactions. Evol Bioinform Online 2006; 2:103 - 20; PMID: 19455205
- Lehnert S, Van Loo P, Thilakarathne PJ, Marynen P, Verbeke G, Schuit FC. Evidence for co-evolution between human microRNAs and Alu-repeats. PLoS One 2009; 4:e4456; http://dx.doi.org/10.1371/journal.pone.0004456; PMID: 19209240
- Smalheiser NR, Torvik VI. A population-based statistical approach identifies parameters characteristic of human microRNA-mRNA interactions. BMC Bioinformatics 2004; 5:139; http://dx.doi.org/10.1186/1471-2105-5-139; PMID: 15453917
- Liu WM, Chu WM, Choudary PV, Schmid CW. Cell stress and translational inhibitors transiently increase the abundance of mammalian SINE transcripts. Nucleic Acids Res 1995; 23:1758 - 65; http://dx.doi.org/10.1093/nar/23.10.1758; PMID: 7784180
- Panning B, Smiley JR. Activation of RNA polymerase III transcription of human Alu repetitive elements by adenovirus type 5: requirement for the E1b 58-kilodalton protein and the products of E4 open reading frames 3 and 6. Mol Cell Biol 1993; 13:3231 - 44; PMID: 7684492
- Panning B, Smiley JR. Activation of RNA polymerase III transcription of human Alu elements by herpes simplex virus. Virology 1994; 202:408 - 17; http://dx.doi.org/10.1006/viro.1994.1357; PMID: 8009851
- Mariner PD, Walters RD, Espinoza CA, Drullinger LF, Wagner SD, Kugel JF, Goodrich JA. Human Alu RNA is a modular transacting repressor of mRNA transcription during heat shock. Mol Cell 2008; 29:499 - 509; http://dx.doi.org/10.1016/j.molcel.2007.12.013; PMID: 18313387
- Yakovchuk P, Goodrich JA, Kugel JF. B2 RNA and Alu RNA repress transcription by disrupting contacts between RNA polymerase II and promoter DNA within assembled complexes. Proc Natl Acad Sci U S A 2009; 106:5569 - 74; http://dx.doi.org/10.1073/pnas.0810738106; PMID: 19307572
- Wang J, Geesman GJ, Hostikka SL, Atallah M, Blackwell B, Lee E, Cook PJ, Pasaniuc B, Shariat G, Halperin E, et al. Inhibition of activated pericentromeric SINE/Alu repeat transcription in senescent human adult stem cells reinstates self-renewal. Cell Cycle 2011; 10:3016 - 30; http://dx.doi.org/10.4161/cc.10.17.17543; PMID: 21862875
- Conley AB, Miller WJ, Jordan IK. Human cis natural antisense transcripts initiated by transposable elements. Trends Genet 2008; 24:53 - 6; http://dx.doi.org/10.1016/j.tig.2007.11.008; PMID: 18192066
- Osato N, Suzuki Y, Ikeo K, Gojobori T. Transcriptional interferences in cis natural antisense transcripts of humans and mice. Genetics 2007; 176:1299 - 306; http://dx.doi.org/10.1534/genetics.106.069484; PMID: 17409075
- Fire A. RNA-triggered gene silencing. Trends Genet 1999; 15:358 - 63; http://dx.doi.org/10.1016/S0168-9525(99)01818-1; PMID: 10461204
- Hu Q, Tanasa B, Trabucchi M, Li W, Zhang J, Ohgi KA, Rose DW, Glass CK, Rosenfeld MG. DICER- and AGO3-dependent generation of retinoic acid-induced DR2 Alu RNAs regulates human stem cell proliferation. Nat Struct Mol Biol 2012; 19:1168 - 75; http://dx.doi.org/10.1038/nsmb.2400; PMID: 23064648
- Chu WM, Ballard R, Carpick BW, Williams BR, Schmid CW. Potential Alu function: regulation of the activity of double-stranded RNA-activated kinase PKR. Mol Cell Biol 1998; 18:58 - 68; PMID: 9418853
- Rubin CM, Kimura RH, Schmid CW. Selective stimulation of translational expression by Alu RNA. Nucleic Acids Res 2002; 30:3253 - 61; http://dx.doi.org/10.1093/nar/gkf419; PMID: 12136107
- Bovia F, Fornallaz M, Leffers H, Strub K. The SRP9/14 subunit of the signal recognition particle (SRP) is present in more than 20-fold excess over SRP in primate cells and exists primarily free but also in complex with small cytoplasmic Alu RNAs. Mol Biol Cell 1995; 6:471 - 84; http://dx.doi.org/10.1091/mbc.6.4.471; PMID: 7542942
- Chang DY, Hsu K, Maraia RJ. Monomeric scAlu and nascent dimeric Alu RNAs induced by adenovirus are assembled into SRP9/14-containing RNPs in HeLa cells. Nucleic Acids Res 1996; 24:4165 - 70; http://dx.doi.org/10.1093/nar/24.21.4165; PMID: 8932367
- Hoffman Y, Dahary D, Bublik DR, Oren M, Pilpel Y. The majority of endogenous microRNA targets within Alu elements avoid the microRNA machinery. Bioinformatics 2013; 29:894 - 902; http://dx.doi.org/10.1093/bioinformatics/btt044; PMID: 23361327
- Gong C, Maquat LE. “Alu”strious long ncRNAs and their role in shortening mRNA half-lives. Cell Cycle 2011; 10:1882 - 3; http://dx.doi.org/10.4161/cc.10.12.15589; PMID: 21487233
- Gong C, Maquat LE. lncRNAs transactivate STAU1-mediated mRNA decay by duplexing with 3′ UTRs via Alu elements. Nature 2011; 470:284 - 8; http://dx.doi.org/10.1038/nature09701; PMID: 21307942