Abstract
Cajal bodies (CBs) are distinct nuclear bodies physically and functionally associated with the nucleolus. In addition to their traditional function in coordinating maturation of certain nuclear RNAs, CBs participate in cell cycle regulation, development, and regulation of stress responses. A key “signature” component of CBs is coilin, the scaffolding protein essential for CB formation and function. Using an RNA silencing (loss-of-function) approach, we describe here new phenomena whereby coilin also affects, directly or indirectly, a variety of interactions between host plants and viruses that have RNA or DNA genomes. Moreover, the effects of coilin on these interactions are manifested differently: coilin contributes to plant defense against tobacco rattle virus (tobravirus), tomato black ring virus (nepovirus), barley stripe mosaic virus (hordeivirus), and tomato golden mosaic virus (begomovirus). In contrast, with potato virus Y (potyvirus) and turnip vein clearing virus (tobamovirus), coilin serves to increase virus pathogenicity. These findings show that interactions with coilin (or CBs) may involve diverse mechanisms with different viruses and that these mechanisms act at different phases of virus infection. Thus, coilin (CBs) has novel, unexpected natural functions that may be recruited or subverted by plant viruses for their own needs or, in contrast, are involved in plant defense mechanisms that suppress host susceptibility to the viruses.
Keywords: :
Introduction
Viruses are intracellular pathogens with small genomes. They therefore rely on host cell functions and cellular machinery to aid their own replication. Elucidating the interaction between host cell and virus components is critical for understanding the mechanisms of virus infection and for the development of novel strategies to control virus diseases.
The cell nucleus is a highly dynamic organelle of eukaryotic cells that regulates major cellular events, including ribosome subunit biogenesis, mRNA synthesis and processing, and DNA replication.Citation1 Since the nucleus plays a major role in plant cell function, it is conceivable that many viruses, whether or not they multiply in the nucleus of infected cells, encode components that may interact with this organelle and its compartments. Indeed, an increasing number of reports show that various animal and plant virus components interact with the nucleolus (for a review see refs. Citation2–Citation4). The nucleolus is a prominent, dynamic subnuclear domain traditionally thought of as the site of rDNA transcription, rRNA processing, and ribosome assembly.Citation5-Citation7 It has also been implicated in many other important cell functions such as cell cycle regulation, gene silencing, telomerase activity, senescence, stress responses, and biogenesis of multiple kinds of ribonucleoprotein (RNP) particles.Citation7-Citation9 In addition to these diverse activities, the nucleolus also plays a crucial role in the infection cycle of several viruses, which is perhaps not surprising considering its various kinds of interaction with viral proteins.Citation2 These associations with the nucleolus are thought to be a universal “pan-virus” phenomenon, such that plant viruses do not differ from other eukaryotic viruses in this regard.Citation4
One plant virus that targets the nucleolus is groundnut rosette virus (GRV, an umbravirus). The ability of its movement (ORF3) protein to transport viral RNA over long distances through the phloem, the specialized plant vascular system, has been shown to depend strictly on its trafficking through the nucleolus and interacting with fibrillarin, the major nucleolar protein.Citation10-Citation12 This interaction is essential for re-localization of some fibrillarin from the nucleolus to the cytoplasm, and also for assembly of cytoplasmic umbraviral RNP particles that are required for the long-distance systemic spread of the virus.Citation10-Citation12 The multifunctional viral genome-linked protein (VPg) encoded by another plant virus, potato virus A (PVA, a potyvirus), has also been shown to accumulate in the nucleolus and interact with fibrillarin.Citation13 However, in contrast to the GRV scenario, with PVA these effects appear to counteract plant host defense (e.g., to decrease RNA silencing rather than facilitate systemic virus spread). This illustrates the diversity of regulatory interactions between viruses and the nucleolus. Many other plant virus proteins have also been shown to localize to the nucleolus and interact with its components.Citation4,Citation14-Citation16 While many of these interactions have benefits for the virus lifecycle, some nucleolar associations can be detrimental to virus replication. For example, nucleolin, one of the nucleolar proteins, binds to the 3′ non-coding region of tomato bushy stunt virus (TBSV, tombusvirus) RNACitation17 and significantly inhibits the viral RNA replication, presumably representing an innate resistance system of a plant host.
Much less is known about virus interactions with other sub-nuclear structures, such as Cajal bodies (CBs). CBs are physically and functionally associated with the nucleolus and participate in the maturation of certain nuclear RNAs, the assembly, modification, and transport of different classes of RNP particles (e.g., spliceosomal small nuclear RNPs, small nucleolar RNPs, telomerase, and U7 snRNP) and also in histone mRNA 3′-end processing (for reviews see refs. Citation18–Citation27). Although CBs participate in defined activities, they have a broad impact on cellular processes. For example, it has been suggested that along with the nucleolus, CBs participate in cell cycle regulation, development, and regulation of stress responses.Citation28-Citation32 One of the key “signature” components of CBs is coilin, which is the structural scaffolding protein essential for CB formation and functional activity.Citation33-Citation40 Coilin is also present in the nucleoplasm in a diffuse form.Citation2,Citation41,Citation42 Although the function of nucleoplasmic coilin is obscure, it has been shown to interact with major components of the DNA repair process (Ku proteins) and can inhibit in vitro non-homologous DNA end-joining.Citation43 This suggests that nucleoplasmic coilin may play a role in stress response pathways such as those activated by DNA damage.Citation43
With regard to the involvement of CBs in virus infections, the available information is confined to a few reports. For example, prior to its accumulation in nucleoli, the GRV ORF3 protein hijacks CBs as vehicles to enter the nucleolus.Citation10-Citation12 In addition, herpes simplex virus 1 and adenoviruses direct coilin and other CB components to the periphery of viral replication centers, where they are involved in processing of late-phase viral transcripts.Citation44 To assess the generality of such findings and to understand the functional role and significance of coilin and CBs in development of virus infections, we have examined the effect of silencing coilin expression on plant interactions with diverse viruses. In this work we used viruses belonging to six taxonomically unrelated groups: five RNA viruses, including tobacco rattle virus (TRV, genus Tobravirus), barley stripe mosaic virus (BSMV, genus Hordeivirus), potato virus Y (PVY, genus Potyvirus), tomato black ring virus (TBRV, genus Nepovirus),
and turnip vein clearing virus (TVCV, genus Tobamovirus), as well as tomato golden mosaic virus (TGMV, genus Begomovirus), a DNA virus (). Remarkably, in comparison to WT plants it was found that coilin deficient lines have altered responses to virus infection, such that some viruses have enhanced infection in the KD lines (TRV, TBRV, BSMV, and TGMV), whereas other viruses are much less pathogenic (PVY and TVCV); coilin deficiency therefore results in two distinct and opposite effects on virus infection ().
Table 1. Properties of viruses studied in this work and effects of coilin KD
Results
Transgenic silencing of the coilin homolog gene in Nicotiana benthamiana and N. tabacum (tobacco) plants
Collier et al.Citation33 identified an Arabidopsis gene (At1g13030; Atcoilin) encoding a definite (though distant) homolog of the vertebrate coilin gene, both on the basis of functional correspondence (being required for CB formation) and structural homology. Homozygous ncb-1 Arabidopsis mutants have lesions in the coilin gene and are completely viable (under normal growth conditions), even though CBs are not produced.Citation33 Similarly, the absence of CBs in coilin-deficient mutants of Drosophila did not affect organism viability.Citation35 We have tested the effects of transgenic silencing of coilin expression in N. benthamiana and tobacco plants (highly susceptible model hosts for many plant viruses) on their interactions with viruses. Coilin-silenced (knocked down, KD) transgenic plants were generated by transgenic expression of two independent hairpin RNA constructs, which were designed to avoid “off-target” silencing.
To design these hairpin RNAi constructs, we performed a database search using the Arabidopsis coilin sequence (At1g13030) to find the coding sequence of the coilin gene of species in the Solanaceae. Although no extensive sequences corresponding to a coilin homolog were identified in Nicotiana species, a potato-expressed sequence tag (EST) was found. This was used for further database searches that revealed ESTs from N. tabacum (FG161029, FG191914) and N. benthamiana (EH370342), which overlapped. Primers were designed to amplify the N. benthamiana coilin gene. Subsequently a ~900 bp fragment of this gene was isolated and sequenced. This sequence has been submitted to DDBJ, EMBL, and GenBank databases under accession number KF482867. Although this sequence was shorter than the corresponding Arabidopsis coilin gene, it was long enough to design two independent hairpin RNAi constructs (coilin 5′ and coilin 3′). Taking into account that only one coilin gene copy is present in the genomes of Arabidopsis,Citation33 diploid potato, and tomato (our unpublished data), it seems possible to assume the coilin in Nicotiana spp. is also produced by a single-copy gene (which then is a target for silencing). The sequences of these two independent hairpin fragments of the coilin ORF were screened using the siRNA scan website (http://bioinfo2.noble.org/RNAiScan.htm)Citation48 to identify 21 nt stretches that have homology with other genes and thus have potential for “off-target” silencing. No sequence matches were found in either fragment in any of the data sets (N. benthamiana, tobacco, tomato, and Arabidopsis). It is therefore unlikely that the sequences would silence genes other than the coilin gene. However, considering the lack of full-genome sequences of tobacco and N. benthamiana,Citation49 a functional complementation assay was performed to determine whether the effects observed on RNAi were due to specific silencing of the coilin gene, as described below.
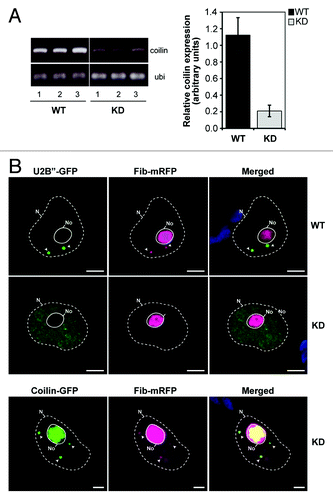
Tests using sqRT-PCR revealed an approximately 80% reduction in the coilin transcript level in the N. benthamiana KD lines (). Similar reductions in coilin accumulation were observed in the tobacco KD transgenic lines, suggesting a high level of similarity between tobacco and N. benthamiana coilin-encoding nucleotide sequences. Consistent with these data, no CBs were observed in the vast majority of nuclei in coilin KD plants (303 of 311 in N. benthamiana and 121 of 125 in N. tabacum), whereas in wild type (WT) plants one, less frequently two, or occasionally more CBs were normally present in each nucleus (). To confirm that the lack of CBs observed was due to specific silencing of the coilin gene, we performed a functional complementation assay. Agrobacterium-mediated expression of the heterologous Arabidopsis coilin gene in the KD transgenic leaves restored the WT phenotype (reappearance of CBs), indicating specificity of RNA silencing (). Notably, no 21 nt regions of homology were found between either of the two RNAi constructs used for generation of transgenic plants and the Arabidopsis coilin ORF. In spite of the significant reduction of coilin expression and the lack of CBs, coilin KD plants did not show any discernibly altered phenotype under normal glasshouse conditions. These results were typical of all our N. benthamiana and tobacco transgenic lines. Most tests used silenced tobacco KD (Nt)1-1 and N. benthamiana KD(Nb)1-1 generated with the RNAi fragment coilin-5′. Although we used viruses that encode silencing suppressors, these did not affect coilin silencing in the KD lines (data not shown).
Figure 1. Transgenic silencing of the coilin gene in N. benthamiana plants. (A) Coilin gene expression levels determined using sqRT-PCR with ubiquitin as a control. PCR image for 30 cycles is shown at the left panel, and PCR signal intensities normalized to ubiquitin levels are indicated at the right panel (data are mean ± SD of four replicates). (B) Effect of transgenic coilin knockdown on the presence of CBs. Confocal laser scanning microscopy of nuclei from WT and KD plants using U2B”-GFP and Fib-mRFP (markers for CBs and for nucleoli and CBs, respectively) shows the absence of CBs in KD leaves (top panel). Restoration of WT phenotype (re-appearance of CBs) was achieved by ectopic expression of A. thaliana coilin-GFP (bottom panel). N, the nucleus; No, the nucleolus. CBs are shown by arrows. Bars, 5 µm. Essentially similar results were obtained in N. tabacum plants.
Viruses with enhanced infection in coilin KD plants: coilin-mediated plant anti-viral defense
In various plants such as N. tabacum (tobacco) or N. benthamiana recently infected with some viruses, such as TRV or TBRV, levels of viral RNA are high, but with time these levels may decline and the infected plants enter the phase known as recovery.Citation50,Citation51 At this stage (about 18 d after infection) symptoms in newly emerging (young) systemically infected leaves are significantly milder, and virus replication may be maintained at low levels.Citation51 The reduction in virus accumulation has been attributed to RNA silencing.Citation51
Remarkably, in contrast to TRV or TBRV-infected WT tobacco plants, KD plants infected with these viruses did not recover from infection and exhibited persistent severe systemic symptoms including curling, dwarfing, leaf malformation, and some necrosis (), suggesting that coilin may be involved in attenuation of (recovery from) TRV and TBRV infections.
Figure 2. Typical systemic symptoms induced by different viruses in WT and coilin KD plants. (A) Symptoms of viruses with pathogenicity enhanced by coilin deficiency: TRV (N. tabacum, at 21 dpi), TBRV (N. tabacum, at 21 dpi), BSMV (N. benthamiana, at 20 dpi), and TGMV (N. benthamiana, at 21 dpi). (B) Symptoms of viruses with pathogenicity reduced by coilin deficiency: TVCV (N. benthamiana, at 26 dpi) and PVY (N. tabacum at 7 dpi).
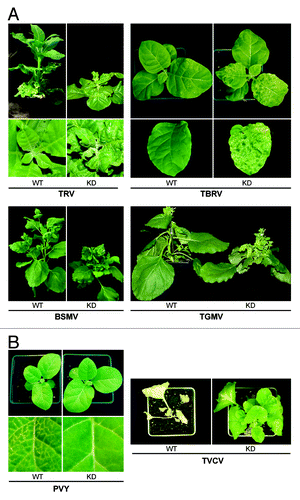
BSMV and TGMV infections in WT N. benthamiana plants do not show any signs of recovery (even 8–10 wk after inoculation), causing persistent mild systemic symptoms. However, as with TRV and TBRV, these viruses, too, induced much more severe systemic symptoms in coilin KD plants (including stunting and leaf malformation) than in WT plants ().
Collectively, these observations imply that coilin is involved in a potential host defense mechanism that decreases host sensitivity to all four viruses: TRV, TBRV, BSMV, and TGMV.
To explore the effect of coilin deficiency on this host defense in more detail, we used TRV (exhibiting recovery from infection in WT plants) and BSMV (showing no recovery). We first compared virus replication rates in a suspension of protoplasts isolated from WT and coilin KD plants (tobacco for TRV and N. benthamiana for BSMV). Notably, protoplasts isolated from coilin-KD plants were still silenced for coilin and contained markedly diminished levels of coilin mRNA (data not shown). Using cultures of TRV and BSMV that express a green fluorescent protein (GFP) reporter gene (TRV-GFPCitation52 and BSMV-GFPCitation53) and confocal laser scanning microscopy (CLSM) we found that the percentage of infected protoplasts was similar for both WT and coilin KD lines (65% vs. 64%, respectively, for TRV and 53% vs. 51%, respectively, for BSMV at 48 h post inoculation [hpi]). Virus accumulation rates in protoplasts of WT and coilin KD lines determined by ELISA were also similar with both viruses (), suggesting that BSMV replication rates in single cells were not altered by the reduced levels of coilin.
Figure 3. Effect of coilin knockdown on TRV and BSMV infection in N. tabacum and N. benthamiana, respectively. (A) Virus accumulation in protoplasts determined by ELISA. Data are mean ± SD of four independent replicate experiments. (B) Accumulation of TRV in inoculated (inoc), old systemic (sys-o, before WT recovery), and newly emerging systemic (sys-n, after WT recovery) leaves and BSMV in inoculated (inoc), old systemic (sys-o), and newly emerging systemic (sys-n) leaves, determined by ELISA. Data are mean ± SD of four independent replicate experiments. (C) Detection (RNA gel blot analysis) of TRV-specific RNA-1 and siRNAs in inoculated (8 dpi) and newly emerging systemic (after recovery, 24 dpi) leaves, and BSMV-specific RNA-α and siRNAs in inoculated (4 dpi) and newly emerging systemic (20 dpi) leaves. Ethidium bromide stained rRNA and 5S rRNA are shown as loading controls for viral RNAs and siRNAs, respectively (the steady-state concentrations of abundant rRNAs including 5S rRNA [which is present in the LMV RNA population] do not depend on virus infection, and they are widely used as loading controls for both viral and siRNAs [e.g., ref. Citation58]). Positions of 24- and 21-nt size markers are indicated.
![Figure 3. Effect of coilin knockdown on TRV and BSMV infection in N. tabacum and N. benthamiana, respectively. (A) Virus accumulation in protoplasts determined by ELISA. Data are mean ± SD of four independent replicate experiments. (B) Accumulation of TRV in inoculated (inoc), old systemic (sys-o, before WT recovery), and newly emerging systemic (sys-n, after WT recovery) leaves and BSMV in inoculated (inoc), old systemic (sys-o), and newly emerging systemic (sys-n) leaves, determined by ELISA. Data are mean ± SD of four independent replicate experiments. (C) Detection (RNA gel blot analysis) of TRV-specific RNA-1 and siRNAs in inoculated (8 dpi) and newly emerging systemic (after recovery, 24 dpi) leaves, and BSMV-specific RNA-α and siRNAs in inoculated (4 dpi) and newly emerging systemic (20 dpi) leaves. Ethidium bromide stained rRNA and 5S rRNA are shown as loading controls for viral RNAs and siRNAs, respectively (the steady-state concentrations of abundant rRNAs including 5S rRNA [which is present in the LMV RNA population] do not depend on virus infection, and they are widely used as loading controls for both viral and siRNAs [e.g., ref. Citation58]). Positions of 24- and 21-nt size markers are indicated.](/cms/asset/09816c1f-f5c0-4e9a-b4f4-9480b3f1618a/kncl_a_10928315_f0003.gif)
Next, we analyzed virus accumulation rates in whole leaves of TRV and BSMV-infected plants (tobacco for TRV and N. benthamiana for BSMV). With regard to the TRV observations, the levels of virus detected in the upper (young, newly emerging) symptomatic leaves of the KD plants were much greater than those detected in the corresponding (recovered) leaves of the WT plants, whereas in the inoculated and lower (old) systemic (symptomatic) leaves, the virus levels were comparable in both KD and WT plants (). These results suggest that the WT and coilin KD tobacco plants are equally susceptible to TRV at the initial stages of infection, however at later time points, only WT plants are able to enter the recovery phase by suppressing virus multiplication in newly emerging leaves. Similar results have been obtained using N. benthamiana KD lines for TRV infection.
In contrast to TRV, essentially higher accumulation levels of BSMV in the N. benthamiana coilin KD lines, compared with WT plants, were observed only at the initial stages of infection, primarily in inoculated leaves (). To examine the effect of coilin on early stages of BSMV infection, we compared the number and size of primary infection (multicellular fluorescent) foci induced by BSMV-GFP and TRV-GFP in coilin KD and WT plants. We detected a substantial increase in the number of infection foci induced by BSMV-GFP in coilin KD lines (44 ± 3 for KD vs. 12 ± 2 for WT, ). However, size distribution and brightness of the BSMV-GFP fluorescent foci were similar in both (WT and KD) lines (), suggesting that the rates of replication (per se) in single cells and cell-to-cell movement of BSMV were not changed by coilin deficiency. It is more likely that the total accumulation of the virus in the inoculated leaves was greater in KD plants due to the increased number of primary infection sites. This may imply that coilin could contribute to some restriction of early (pre-replication) stages of infection such as cell entry, disassembly of virus particles, and initial translation.Citation54 Interestingly, inoculation of the coilin WT plants with BSMV-GFP RNA did not increase their relative susceptibility to infection compared with coilin KD plants, as indicated by lesion number (28 ± 3 for KD vs. 7 ± 2 for WT), suggesting that the uncoating of virus particles during primary infection is not affected by coilin. Alternatively, coilin might be involved in an RNA silencing-based plant defense pathway (see below). No difference in the number (68 ± 8 for KD vs. 75 ± 9 for WT), size, and brightness of primary infection foci induced by TRV-GFP in coilin KD and WT plants was observed ().
Figure 4. Fluorescent foci, induced by BSMV-GFP and TRV-GFP in inoculated leaves of WT and coilin KD plants.
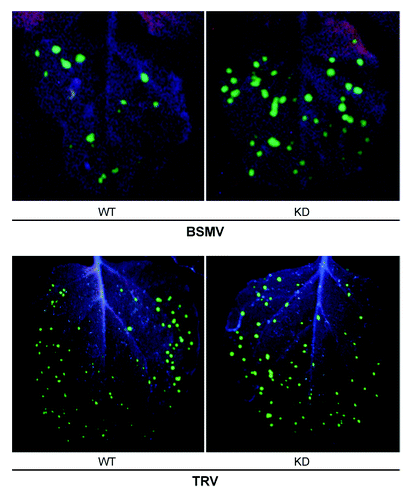
In systemically infected leaves of coilin KD plants, BSMV was first detected two days earlier than in systemic leaves of the WT plants (commonly, 3 d post-inoculation [dpi] for coilin KD lines vs. 5 dpi for WT plants), which might be a result of faster and greater accumulation of the virus in inoculated leaves and/or its accelerated long-distance movement in the KD plants. However, after reaching systemic leaves, pattern and levels of BSMV accumulation in the systemic leaves of coilin KD did not differ from those in WT, suggesting that virus unloading from the vascular transport system as well as virus replication rates in the systemic leaves were not affected by coilin deficiency. Taken together, these results support the idea that coilin can contribute to modulation of plant susceptibility at early stages of BSMV infection by reducing the number of primary inoculated cells that give rise to infection foci. This could eventually delay systemic spread of the virus and restrict its pathogenicity for whole infected (WT) plants, as manifested by mild symptoms ().
However, when BSMV inoculum concentrations were adjusted to give similar lesion numbers in inoculated leaves of WT and KD plants (BSMV inoculum was diluted 5-fold for KD plants), the relative speed of systemic infection was still higher for coilin KD plants (BSMV was detected in systemic leaves at 3 dpi for coilin KD lines vs. 5 dpi for WT plants, the same pattern as with equal concentrations of BSMV inoculum), suggesting that the mechanism of long-distance movement is affected by coilin independently of susceptibility at early stages of infection. At later stages such as 20 dpi, the efficiency of long distance movement in WT and coilin KD plants is likely masked by the similar high magnitude level of BSMV replication in both genotypes, such that, with time, differences in BSMV levels became negligible ().
Collectively, these findings show that coilin (CBs) may control diverse molecular defense mechanisms against the unrelated viruses, TRV and BSMV, which exhibit quite different biological properties (e.g., recovery with TRV and no recovery with BSMV). Indeed, these defense mechanisms may comprise plant responses that contribute to either plant recovery (in the case of TRV) or reduction in the number of initial infection foci (plant susceptibility) in inoculated leaves (as with BSMV).
Is RNA silencing involved in coilin-mediated plant anti-viral defense?
A major mechanism of plant defense against viruses is RNA silencing, whereby the plant targets viral RNA for degradation, which can lead to the recovery phenomenon or compromises the replication and spread of the virus.Citation55,Citation56 In order to overcome this resistance strategy, plant viruses have evolved different silencing suppressors which can block various components of the silencing machinery and permit virus invasion.Citation57 The success of this strategy varies depending on the host plant-virus combination.
Given the role of RNA silencing in plant anti-viral defense, the increase in levels of TRV (in systemic leaves) and BSMV (in inoculated leaves) in coilin KD plants could suggest that this pathway may be compromised as a consequence of the reduced levels of coilin. To test this hypothesis, we analyzed the accumulation of TRV- and BSMV-specific small interfering RNAs (siRNAs), the main hallmark of RNA silencing processes (for a review see refs. Citation55,Citation56). The low-molecular-weight RNA samples isolated from WT and coilin KD plants infected with TRV or BSMV were subjected to northern blot analysis using probes derived from TRV and BSMV RNAs, respectively. Remarkably, in contrast to expectation, the levels of TRV and BSMV specific siRNAs were not reduced in coilin KD plants (). Moreover, they were slightly increased, indicating that silencing initiation was not inhibited in coilin deficient plants. Thus, coilin KD plants are able to trigger specific silencing against TRV or BSMV at levels comparable with WT plants. This suggests that the activity of dicer-like RNase III enzymes (DCL), one of the initial steps of RNA silencing leading to the cleavage of viral dsRNAs into siRNAs,Citation58 was not altered in coilin KD plants. However, in contrast to WT in coilin KD plants, both TRV (in systemic leaves) and BSMV (in inoculated leaves) seem not to be targeted by the induced silencing (or targeted much less efficiently than in WT). Given that KD of coilin enhances TRV and BSMV accumulation, this implies that partial removal of coilin renders TRV and BSMV able to overcome (evade and/or suppress) late steps of the RNA silencing processes.
One possibility is that coilin reduces silencing suppression activity of the TRV 16KCitation59 and BSMV γbCitation60 proteins, the known TRV and BSMV silencing suppressors, implying stronger silencing suppression in the coilin deficient (KD) plants. However, this seems unlikely because levels of TRV 16K- and BSMV γb-mediated suppression of GFP gene silencing were similar in coilin KD and WT plants (Fig. S1). Another possibility could be that coilin itself is required for late stages of some virus silencing pathways (e.g., assembly or activity of specific siRNA-induced silencing complexes, RISCs).Citation61,Citation62 Alternatively, or additionally, coilin may be involved in another previously unrecognized host defense mechanism operating in virus-infected leaves. If so, coilin deficiency (in coilin KD plants) would weaken this defense mechanism, facilitating accumulation of virus RNA to levels that potentially saturate the silencing machinery and thus enabling evasion of TRV (in systemic leaves) and BSMV (in inoculated leaves) from the silencing.
Viruses with impaired infection in coilin KD plants
While coilin KD enhanced TRV, TBRV, TGMV, and BSMV pathogenicity, it had the completely opposite effect on PVY and TVCV, causing attenuation of infection (or significant delay in its development). For example, with PVY it was apparent at 7 dpi that the vein clearing and necrotic symptoms in the systemic leaves of WT tobacco were much more pronounced than those in the coilin KD plants (). Similarly, TVCV induced very severe symptoms in WT N. benthamiana plants often leading to death of plants, whereas symptoms in coilin KD plants were much milder ().
Strong TVCV-induced symptoms in WT plants precluded further analysis of these plants. Therefore, to investigate the reason for symptom attenuation in coilin deficient plants, we used PVY. First, we compared virus replication rates in WT and coilin KD tobacco protoplasts. Infection of protoplasts was assessed by immunofluorescence microscopy using anti-PVY antibody at 48 hpi. The percentage of infected protoplasts was essentially similar for the coilin KD (65%) and WT (60%) lines, whereas virus accumulation rates in coilin KD protoplasts was markedly (approximately 6-fold) lower than in WT protoplasts ().
Figure 5. Effect of coilin knockdown on PVY infection in N. tabacum plants. Accumulation of PVY in protoplasts (A) and in inoculated (inoc) and systemic (sys) leaves (B) determined by ELISA. Data are mean ± SD of four independent replicate experiments. (C) Detection (RNA gel blot analysis) of viral RNA and siRNAs in inoculated (4 dpi) and systemic (8 dpi) leaves. Ethidium bromide (EtBr) stained rRNA and 5S rRNA are shown as loading controls for viral RNAs and siRNAs, respectively. Positions of 24- and 21-nt size markers are indicated.
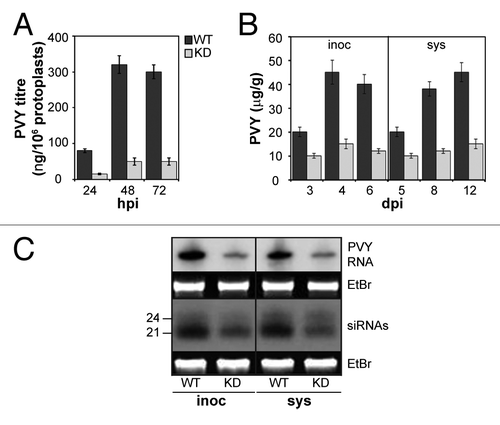
In the next series of experiments, we showed that levels of accumulation of PVY in inoculated leaves of coilin KD plants were also lower than those in WT leaves, although the difference (3–4-fold) was smaller (). However, in spite of the reduced accumulation rates of PVY in inoculated coilin KD plants, systemic spread of PVY to upper non-inoculated systemic leaves was unaffected by coilin deficiency: in both WT and coilin KD plants, the virus was first detected in systemic leaves at 5 dpi as determined by ELISA, suggesting that long-distance movement of PVY was not restricted in the KD plants. Further accumulation of PVY in systemic leaves was also reduced in coilin KD plants (). The molecular basis of this reduction cannot be accounted for by RNA silencing (at least at the DCL activity level) as the amounts of PVY-specific siRNAs detected in coilin KD leaves were not increased in comparison to WT but were slightly reduced (). Moreover, silencing suppression activity mediated by PVY HC-Pro silencing suppressorCitation63 was also not affected in coilin KD lines compared with WT plants (Fig. S1). However, it is possible that a coilin-mediated mechanism may interfere with some other pathways of host anti-PVY defense (perhaps including RISC-related activity or other general defense mechanisms [e.g., mediated by salicylic acid]). Alternatively, coilin could facilitate efficient PVY production by direct effect on the RNA replication process. Collectively, these results suggest that PVY recruits or subverts certain function(s) of coilin to establish more robust systemic infection and facilitate efficient replication of the virus. Essentially similar data have been obtained using N. benthamiana KD lines.
Discussion
Our results have revealed a set of novel phenomena that should lead to much further research. Specifically, we have shown that plant coilin affects, directly or indirectly, the interaction of host plants with RNA- and DNA-containing viruses in six widely different taxonomic groups (). This implies that coilin is likely also to affect many other host-virus interactions. Moreover, the effects of coilin (or CBs) on these interactions are manifested differently in different virus taxa. However, our preliminary data also indicate that not all plant viruses are affected by coilin deficiency (e.g., potato virus X, genus Potexvirus).
Coilin is a major scaffolding protein of CBs and is essential for their formation.Citation33-Citation37 In addition to its structural role, coilin may also be implicated in functional activities ascribed to the CB. Indeed, coilin has recently been found to bind both ssRNA and dsDNA and to possess RNase activity in vitro,Citation38-Citation40 hinting that it may take part in some aspects of CB function (e.g., processing of U2 snRNA primary transcript in a DNA dependent manner as suggested by Broome et al.).Citation38,Citation39 Although coilin is known as the CB signature protein, it is also found in the nucleoplasm. In fact, the majority of coilin is found in the nucleoplasm, not the CB.Citation41 The function of this nucleoplasmic pool of coilin remains unclear but may include a role in some stress response pathways such as those caused by DNA damage.Citation43
Using an RNA silencing (loss-of-function) approach, we demonstrate here that interaction with coilin also affects a variety of plant viruses. It can be assumed that coilin silencing in transgenic plants, described in this work, leads not only to the loss of CBs but also to the reduction of coilin content in the nucleoplasm. Both of these events may potentially affect virus-plant interactions. Data presented here do not allow us to differentiate between these two possibilities, and therefore, both of them are referred to in this paper as a “coilin-mediated mechanism,” implicating involvement of either (1) coilin itself as a functional protein, (2) CBs as an appropriate site for a certain molecular event, dependent on coilin for formation, or (3) both coilin and CBs. Future experiments will aim to discover how coilin (in the nucleoplasm or CBs) can directly or indirectly (through its ability to form CBs) affect virus infections.
As reported previously, GRV recruits CBs as vehicles for delivery of the viral ORF3 protein into the nucleolus,Citation10-Citation12 and herpes simplex virus 1 and adenoviruses re-localize coilin and other CB components to the periphery of viral replication centers, where they are involved in processing of late-phase viral transcripts.Citation44 In this study we have demonstrated that coilin (either itself or through formation of CBs) contributes to plant host defense against four viruses: TRV, TBRV, BSMV, and TGMV at different stages of infection (). In contrast, with PVY and TVCV, coilin (or CBs) presumably is used to increase pathogenicity by facilitating replication of these viruses (). Altogether, these findings show that interactions with coilin (CBs) may involve diverse molecular mechanisms with different viruses, and these act at various phases of the virus infection cycle. Thus coilin (CBs) may have novel, unexpected natural functions that are either recruited by plant viruses for their own needs or, in contrast, are involved in plant defense mechanisms that suppress host susceptibility to the viruses.
These functions may also be involved in other aspects of cellular pathways—in particular, stress responses. Indeed the structure of CBs has been shown to be altered by different types of abiotic stress, such as UV irradiation, heat shock, transcriptional inhibition, osmotic stress, and starvation, implying that CBs may be involved in cellular responses to stress (for a review see ref. Citation32). Knockdown and knockout studies in Arabidopsis and Drosophila models demonstrate that reduced levels of coilin do not result in decreased viability,Citation33,Citation35 suggesting that neither coilin itself nor CBs are essential for these organisms under normal conditions. What, then, is the functional role of coilin (and CBs) in these species? A possibility is that they are essential under stress conditions, providing defense against abiotic stress and pathogen (virus) attack. In addition, recent studies in zebrafish and mouse models suggest that the formation of CBs may play an essential role in snRNP processing during development (embryogenesis).Citation30-Citation32
Although the underlying mechanisms remain to be revealed, these observations suggest there is an interplay between coilin (and CB) functions on one hand and, on the other hand, pathways involved in host defense, stress tolerance, and development, which are known to overlap.Citation32 With regard to plant viruses, the ultimate pattern of virus infection is determined by the race between host defense and virus replication, spread, and counter-defense activities. As shown here, functions of coilin (CBs) can potentially contribute to either side in this race, implying that the mechanisms involved are complex and intertwined. One possible activity of coilin may be a direct interaction with viral proteins, as was shown for a virus protein encoded by poa semilatent virus, which is closely related to BSMV.Citation64
In conclusion, the involvement of coilin (CBs) in various plant-virus interactions represents novel phenomena that expand knowledge of the biology of CBs and coilin. In recent years the importance and relations between plant viruses and the nucleolus have been well established (for a review see ref. Citation4). In light of our findings, these relations should now be extended to include CBs and their signature protein, coilin. Indeed, this paper defines a framework for further studies of molecular and cellular functions of coilin (and CBs) in response to the effects of infection by diverse viruses and, quite possibly, to different other kinds of stress.
Materials and Methods
Cloning of N. benthamiana coilin cDNA
To isolate a cDNA encoding a homolog of coilin from N. benthamiana, primers were designed to amplify a fragment of ~900 bp using the Expand Hi-Fidelity PCR system (Roche) based on the sequence of an EST of N. tabacum (FG161029), which aligned and overlapped with a potato EST (DR035929). The PCR product was cloned into the pGEMTeasy TA cloning vector (Promega) and sequenced. The sequence data has been submitted to the DDBJ/EMBL/GenBank databases under accession number KF482867.
Transgenic plants
For transgenic silencing, the coilin 5′ (nt 1–309 in ref. accession) and coilin 3′ (nt 464–882 in ref. accession) cDNA fragments were cloned into the pDONR207 vector and transferred to a Gateway compatible version of the binary plasmid pFGC5941. This vector contains Gateway cassettes either side of the chalcone synthase intron. The cloned RNAi fragments were thus cloned in opposite orientations facilitating the formation of a hairpin loop. The resultant plasmid was electroporated into A. tumefaciens and used to transform N. benthamiana and N. tabacum Samsun (NN) as described.Citation65 RT-PCR analysis of the accumulation of coilin mRNA was performed by using the reciprocal primer combination, e.g., for the 5′ transgenics the 3′ oligos were used, and vice versa. First-strand cDNA was primed with oligo dT using SuperScript II reverse transcriptase (Invitrogen) according to the manufacturer's recommendations and used as a template for PCR product amplification through 25, 30, 35, 40, and 45 cycles. The amounts of PCR-generated fragments were analyzed and quantified by using the Intelligent Quantifier, version 2.5.0 (BioImage). A primer set for amplification of a fragment of ubiquitin mRNA (100 nt) was used as a control.
Confocal laser scanning microscopy
Localization of U2B”-GFP and coilin-GFP (CB markersCitation11), and Fibrillarin-mRFP (Fib-mRFPCitation11) were monitored using a Leica TCSSP2 AOBS confocal microscope, at 48–72 h after agroinfiltration. mRFP was imaged using 561 nm excitation with emissions between 600 and 630 nm. GFP was imaged using 488 nm excitation with emissions between 500–530 nm.
Viruses and virus-based constructs
TRV, BSMV, PVY, TBRV, TGMV, and TVCV were used in this study. PVY and TBRV were maintained as stock isolates by repeated passage in N. benthamiana plants and used for mechanical inoculation. The TRV and BSMV infectious cDNA clones were described previously.Citation52,Citation53 To inoculate plants with TRV and TVCV, Agrobacterium cultures expressing full-length virus constructs under control of the 35 S promoter were infiltrated into leaves of N. benthamiana.Citation66 Preparation of BSMV cDNA template, in vitro RNA transcription using the mMessage mMachine T7 kit (Ambion), and inoculation of plants were as described earlier.Citation67 TGMV plasmids (p0191 and p0192) were mixed in a 1:1 ratio and mechanically inoculated onto plants as described previouslyCitation68 using 1.25 µg of each plasmid per leaf. TRV (TRV-GFP)Citation52 and BSMV (γ-GFP-2A-γb; BSMV-GFP)Citation52 constructs that express a GFP reporter were described by MacFarlane and PopovichCitation52 and Lawrence and Jackson,Citation53 respectively.
Preparation and transfection of protoplasts
Protoplasts were isolated from fully expanded mature leaves of N. benthamiana and N. tabacum as described earlier.Citation69 For inoculation to the pellet of 1 × 106 protoplasts, 5 µg of virions in 25 µl of ice-cold water and 1 ml of 40% polyethylene glycol (MW 6000) in 9% mannitol were added. Following a 30 min incubation, the protoplasts were washed and incubated in Murashige and Skoog media supplemented with 9% mannitol at 23–24 °C under continuous illumination. Virus accumulation was analyzed by ELISA as described below. The percentage of infected protoplasts was determined using TRV-GFP and BSMV-GFP constructs (for TRV and BSMV) or by immunofluorescence staining of the protoplasts with fluorescein isothiocyanate-conjugated with PVY antibodies (for PVY), respectively. All protoplast transfections were performed four times.
Viral protein and RNA analysis
The accumulation of TRV, BSMV, and PVY antigens was assessed and quantified by ELISA as described previously.Citation65 Reproducible results were obtained for four independent experiments and data presented are mean ± SD. Total RNA was extracted from 1–2 g of leaf tissue using TRI REAGENT (Sigma) according to the manufacturer's recommendations. High-molecular weight (HMW) and low-molecular weight (LMW) RNAs were isolated and separated as described.Citation65 The HMW and LMW RNAs were resolved by electrophoresis in agarose and polyacrylamide/7M urea gels respectively, and then electrotransferred to Hybond N membrane and UV cross-linked using a StrataLinker (Stratagene). [32P]-labeled RNA probes corresponding to 5′ terminal fragments of TRV RNA1, BSMV RNAα, and PVY RNA were generated using the mMESSAGE mMACHINE T7 kit (Ambion Inc) with a random-priming DNA labeling system (Invitrogen). As a loading control, equal fractions of each sample were resolved on a 1% agarose gel and stained with ethidium bromide (EtBr). Similar results were obtained in four independent experiments.
Additional material
Download Zip (319.4 KB)Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Accession Number
The sequence has been submitted to the DDBJ/EMBL/GenBank databases under accession number KF482867.
Acknowledgments
The authors would like to thank Prof John WS Brown and Dr Christophe Lacomme for helpful and critical discussions. We would also like to thank Prof Andrew O Jackson for provision of the BSMV constructs. This work was funded by Scottish Government Rural and Environmental Science and Analytical Services Division (JS, AJL, and MT) and was also partially supported by Russian Ministry of Education and Science (contract 02.740.11.5145).
References
- Trinkle-Mulcahy L, Lamond AI. Toward a high-resolution view of nuclear dynamics. Science 2007; 318:1402 - 7; http://dx.doi.org/10.1126/science.1142033; PMID: 18048679
- Hiscox JA. RNA viruses: hijacking the dynamic nucleolus. Nat Rev Microbiol 2007; 5:119 - 27; http://dx.doi.org/10.1038/nrmicro1597; PMID: 17224921
- Greco A. Involvement of the nucleolus in replication of human viruses. Rev Med Virol 2009; 19:201 - 14; http://dx.doi.org/10.1002/rmv.614; PMID: 19399920
- Taliansky ME, Brown JWS, Rajamäki ML, Valkonen JP, Kalinina NO. Involvement of the plant nucleolus in virus and viroid infections: parallels with animal pathosystems. Adv Virus Res 2010; 77:119 - 58; http://dx.doi.org/10.1016/B978-0-12-385034-8.00005-3; PMID: 20951872
- Olson MOJ. Sensing cellular stress: another new function for the nucleolus?. Sci STKE 2004; 2004:pe10; PMID: 15026578
- Rubbi CP, Milner J. Disruption of the nucleolus mediates stabilization of p53 in response to DNA damage and other stresses. EMBO J 2003; 22:6068 - 77; http://dx.doi.org/10.1093/emboj/cdg579; PMID: 14609953
- Sirri V, Urcuqui-Inchima S, Roussel P, Hernandez-Verdun D. Nucleolus: the fascinating nuclear body. Histochem Cell Biol 2008; 129:13 - 31; http://dx.doi.org/10.1007/s00418-007-0359-6; PMID: 18046571
- Boisvert F-M, van Koningsbruggen S, Navascués J, Lamond AI. The multifunctional nucleolus. Nat Rev Mol Cell Biol 2007; 8:574 - 85; http://dx.doi.org/10.1038/nrm2184; PMID: 17519961
- Olson MOJ, Dundr M. The moving parts of the nucleolus. Histochem Cell Biol 2005; 123:203 - 16; http://dx.doi.org/10.1007/s00418-005-0754-9; PMID: 15742198
- Kim SH, Macfarlane S, Kalinina NO, Rakitina DV, Ryabov EV, Gillespie T, Haupt S, Brown JWS, Taliansky M. Interaction of a plant virus-encoded protein with the major nucleolar protein fibrillarin is required for systemic virus infection. Proc Natl Acad Sci U S A 2007; 104:11115 - 20; http://dx.doi.org/10.1073/pnas.0704632104; PMID: 17576925
- Kim SH, Ryabov EV, Kalinina NO, Rakitina DV, Gillespie T, MacFarlane S, Haupt S, Brown JWS, Taliansky M. Cajal bodies and the nucleolus are required for a plant virus systemic infection. EMBO J 2007; 26:2169 - 79; http://dx.doi.org/10.1038/sj.emboj.7601674; PMID: 17410203
- Canetta E, Kim SH, Kalinina NO, Shaw J, Adya AK, Gillespie T, Brown JWS, Taliansky M. A plant virus movement protein forms ringlike complexes with the major nucleolar protein, fibrillarin, in vitro.. J Mol Biol 2008; 376:932 - 7; http://dx.doi.org/10.1016/j.jmb.2007.12.039; PMID: 18199452
- Rajamäki M-L, Valkonen JPT. Control of nuclear and nucleolar localization of nuclear inclusion protein a of picorna-like Potato virus A in Nicotiana species. Plant Cell 2009; 21:2485 - 502; http://dx.doi.org/10.1105/tpc.108.064147; PMID: 19700632
- Wright KM, Cowan GH, Lukhovitskaya NI, Tilsner J, Roberts AG, Savenkov EI, Torrance L. The N-terminal domain of PMTV TGB1 movement protein is required for nucleolar localization, microtubule association, and long-distance movement. Mol Plant Microbe Interact 2010; 23:1486 - 97; http://dx.doi.org/10.1094/MPMI-05-10-0105; PMID: 20923354
- Torrance L, Wright KM, Crutzen F, Cowan GH, Lukhovitskaya NI, Bragard C, Savenkov EI. Unusual features of pomoviral RNA movement. Front Microbiol 2011; 2:259; http://dx.doi.org/10.3389/fmicb.2011.00259; PMID: 22203822
- Semashko MA, González I, Shaw J, Leonova OG, Popenko VI, Taliansky ME, Canto T, Kalinina NO. The extreme N-terminal domain of a hordeivirus TGB1 movement protein mediates its localization to the nucleolus and interaction with fibrillarin. Biochimie 2012; 94:1180 - 8; http://dx.doi.org/10.1016/j.biochi.2012.02.005; PMID: 22349738
- Jiang Y, Li Z, Nagy PD. Nucleolin/Nsr1p binds to the 3′ noncoding region of the tombusvirus RNA and inhibits replication. Virology 2010; 396:10 - 20; http://dx.doi.org/10.1016/j.virol.2009.10.007; PMID: 19861225
- Carmo-Fonseca M. New clues to the function of the Cajal body. EMBO Rep 2002; 3:726 - 7; http://dx.doi.org/10.1093/embo-reports/kvf154; PMID: 12151329
- Cioce M, Lamond AI. Cajal bodies: a long history of discovery. Annu Rev Cell Dev Biol 2005; 21:105 - 31; http://dx.doi.org/10.1146/annurev.cellbio.20.010403.103738; PMID: 16212489
- Gall JG. Cajal bodies: the first 100 years. Annu Rev Cell Dev Biol 2000; 16:273 - 300; http://dx.doi.org/10.1146/annurev.cellbio.16.1.273; PMID: 11031238
- Jády BE, Bertrand E, Kiss T. Human telomerase RNA and box H/ACA scaRNAs share a common Cajal body-specific localization signal. J Cell Biol 2004; 164:647 - 52; http://dx.doi.org/10.1083/jcb.200310138; PMID: 14981093
- Jády BE, Richard P, Bertrand E, Kiss T. Cell cycle-dependent recruitment of telomerase RNA and Cajal bodies to human telomeres. Mol Biol Cell 2006; 17:944 - 54; http://dx.doi.org/10.1091/mbc.E05-09-0904; PMID: 16319170
- Kiss AM, Jády BE, Darzacq X, Verheggen C, Bertrand E, Kiss T. A Cajal body-specific pseudouridylation guide RNA is composed of two box H/ACA snoRNA-like domains. Nucleic Acids Res 2002; 30:4643 - 9; http://dx.doi.org/10.1093/nar/gkf592; PMID: 12409454
- Kiss T. Biogenesis of small nuclear RNPs. J Cell Sci 2004; 117:5949 - 51; http://dx.doi.org/10.1242/jcs.01487; PMID: 15564372
- Ogg SC, Lamond AI. Cajal bodies and coilin--moving towards function. J Cell Biol 2002; 159:17 - 21; http://dx.doi.org/10.1083/jcb.200206111; PMID: 12379800
- Schümperli D, Pillai RS. The special Sm core structure of the U7 snRNP: far-reaching significance of a small nuclear ribonucleoprotein. Cell Mol Life Sci 2004; 61:2560 - 70; http://dx.doi.org/10.1007/s00018-004-4190-0; PMID: 15526162
- Bassett C. Cajal bodies and plant RNA metabolism. CRC Crit Rev Plant Sci 2012; 31:258 - 70; http://dx.doi.org/10.1080/07352689.2011.645431
- Andrade LE, Tan EM, Chan EK. Immunocytochemical analysis of the coiled body in the cell cycle and during cell proliferation. Proc Natl Acad Sci U S A 1993; 90:1947 - 51; http://dx.doi.org/10.1073/pnas.90.5.1947; PMID: 8446613
- Carmo-Fonseca M, Ferreira J, Lamond AI. Assembly of snRNP-containing coiled bodies is regulated in interphase and mitosis--evidence that the coiled body is a kinetic nuclear structure. J Cell Biol 1993; 120:841 - 52; http://dx.doi.org/10.1083/jcb.120.4.841; PMID: 7679389
- Strzelecka M, Oates AC, Neugebauer KM. Dynamic control of Cajal body number during zebrafish embryogenesis. Nucleus 2010; 1:96 - 108; PMID: 21327108
- Walker MP, Tian L, Matera AG. Reduced viability, fertility and fecundity in mice lacking the cajal body marker protein, coilin. PLoS One 2009; 4:e6171; http://dx.doi.org/10.1371/journal.pone.0006171; PMID: 19587784
- Boulon S, Westman BJ, Hutten S, Boisvert F-M, Lamond AI. The nucleolus under stress. Mol Cell 2010; 40:216 - 27; http://dx.doi.org/10.1016/j.molcel.2010.09.024; PMID: 20965417
- Collier S, Pendle A, Boudonck K, van Rij T, Dolan L, Shaw P. A distant coilin homologue is required for the formation of cajal bodies in Arabidopsis. Mol Biol Cell 2006; 17:2942 - 51; http://dx.doi.org/10.1091/mbc.E05-12-1157; PMID: 16624863
- Lemm I, Girard C, Kuhn AN, Watkins NJ, Schneider M, Bordonné R, Lührmann R. Ongoing U snRNP biogenesis is required for the integrity of Cajal bodies. Mol Biol Cell 2006; 17:3221 - 31; http://dx.doi.org/10.1091/mbc.E06-03-0247; PMID: 16687569
- Liu J-L, Wu Z, Nizami Z, Deryusheva S, Rajendra TK, Beumer KJ, Gao H, Matera AG, Carroll D, Gall JG. Coilin is essential for Cajal body organization in Drosophila melanogaster. Mol Biol Cell 2009; 20:1661 - 70; http://dx.doi.org/10.1091/mbc.E08-05-0525; PMID: 19158395
- Strzelecka M, Trowitzsch S, Weber G, Lührmann R, Oates AC, Neugebauer KM. Coilin-dependent snRNP assembly is essential for zebrafish embryogenesis. Nat Struct Mol Biol 2010; 17:403 - 9; http://dx.doi.org/10.1038/nsmb.1783; PMID: 20357773
- Tucker KE, Berciano MT, Jacobs EY, LePage DF, Shpargel KB, Rossire JJ, Chan EK, Lafarga M, Conlon RA, Matera AG. Residual Cajal bodies in coilin knockout mice fail to recruit Sm snRNPs and SMN, the spinal muscular atrophy gene product. J Cell Biol 2001; 154:293 - 307; http://dx.doi.org/10.1083/jcb.200104083; PMID: 11470819
- Broome HJ, Hebert MD. In vitro RNase and nucleic acid binding activities implicate coilin in U snRNA processing. PLoS One 2012; 7:e36300; http://dx.doi.org/10.1371/journal.pone.0036300; PMID: 22558428
- Broome HJ, Carrero ZI, Douglas HE, Hebert MD. Phosphorylation regulates coilin activity and RNA association. Biol Open 2013; 2:407 - 15; http://dx.doi.org/10.1242/bio.20133863; PMID: 23616925
- Makarov V, Rakitina D, Protopopova A, Yaminsky I, Arutiunian A, Love AJ, Taliansky M, Kalinina N. Plant coilin: structural characteristics and RNA-binding properties. PLoS One 2013; 8:e53571; http://dx.doi.org/10.1371/journal.pone.0053571; PMID: 23320094
- Lam YW, Lyon CE, Lamond AI. Large-scale isolation of Cajal bodies from HeLa cells. Mol Biol Cell 2002; 13:2461 - 73; http://dx.doi.org/10.1091/mbc.02-03-0034; PMID: 12134083
- Platani M, Goldberg I, Swedlow JR, Lamond AI. In vivo analysis of Cajal body movement, separation, and joining in live human cells. J Cell Biol 2000; 151:1561 - 74; http://dx.doi.org/10.1083/jcb.151.7.1561; PMID: 11134083
- Velma V, Carrero ZI, Cosman AM, Hebert MD. Coilin interacts with Ku proteins and inhibits in vitro non-homologous DNA end joining. FEBS Lett 2010; 584:4735 - 9; http://dx.doi.org/10.1016/j.febslet.2010.11.004; PMID: 21070772
- James NJ, Howell GJ, Walker JH, Blair GE. The role of Cajal bodies in the expression of late phase adenovirus proteins. Virology 2010; 399:299 - 311; http://dx.doi.org/10.1016/j.virol.2010.01.013; PMID: 20137801
- Ghazala W, Waltermann A, Pilot R, Winter S, Varrelmann M. Functional characterization and subcellular localization of the 16K cysteine-rich suppressor of gene silencing protein of tobacco rattle virus. J Gen Virol 2008; 89:1748 - 58; http://dx.doi.org/10.1099/vir.0.83503-0; PMID: 18559946
- Sharma P, Ikegami M. Characterization of signals that dictate nuclear/nucleolar and cytoplasmic shuttling of the capsid protein of Tomato leaf curl Java virus associated with DNA β satellite. Virus Res 2009; 144:145 - 53; http://dx.doi.org/10.1016/j.virusres.2009.04.019; PMID: 19409945
- Levy A, Zheng JY, Lazarowitz SG. The tobamovirus Turnip Vein Clearing Virus 30-kilodalton movement protein localizes to novel nuclear filaments to enhance virus infection. J Virol 2013; 87:6428 - 40; http://dx.doi.org/10.1128/JVI.03390-12; PMID: 23536678
- Xu P, Zhang Y, Kang L, Roossinck MJ, Mysore KS. Computational estimation and experimental verification of off-target silencing during posttranscriptional gene silencing in plants. Plant Physiol 2006; 142:429 - 40; http://dx.doi.org/10.1104/pp.106.083295; PMID: 16920874
- Bombarely A, Rosli HG, Vrebalov J, Moffett P, Mueller LA, Martin GB. A draft genome sequence of Nicotiana benthamiana to enhance molecular plant-microbe biology research. Mol Plant Microbe Interact 2012; 25:1523 - 30; http://dx.doi.org/10.1094/MPMI-06-12-0148-TA; PMID: 22876960
- Ratcliff F, Harrison BD, Baulcombe DC. A similarity between viral defense and gene silencing in plants. Science 1997; 276:1558 - 60; http://dx.doi.org/10.1126/science.276.5318.1558; PMID: 18610513
- Ratcliff FG, MacFarlane SA, Baulcombe DC. Gene silencing without DNA. rna-mediated cross-protection between viruses. Plant Cell 1999; 11:1207 - 16; PMID: 10402423
- MacFarlane SA, Popovich AH. Efficient expression of foreign proteins in roots from tobravirus vectors. Virology 2000; 267:29 - 35; http://dx.doi.org/10.1006/viro.1999.0098; PMID: 10648180
- Lawrence DM, Jackson AO. Requirements for cell-to-cell movement of Barley stripe mosaic virus in monocot and dicot hosts. Mol Plant Pathol 2001; 2:65 - 75; http://dx.doi.org/10.1046/j.1364-3703.2001.00052.x; PMID: 20572993
- Hull R. Matthews’ Plant Virology. London: Academic Press: 2002 .
- Baulcombe D. RNA silencing. Trends Biochem Sci 2005; 30:290 - 3; http://dx.doi.org/10.1016/j.tibs.2005.04.012; PMID: 15950871
- Voinnet O. Induction and suppression of RNA silencing: insights from viral infections. Nat Rev Genet 2005; 6:206 - 20; http://dx.doi.org/10.1038/nrg1555; PMID: 15703763
- Burgyán J, Havelda Z. Viral suppressors of RNA silencing. Trends Plant Sci 2011; 16:265 - 72; http://dx.doi.org/10.1016/j.tplants.2011.02.010; PMID: 21439890
- Deleris A, Gallego-Bartolome J, Bao J, Kasschau KD, Carrington JC, Voinnet O. Hierarchical action and inhibition of plant Dicer-like proteins in antiviral defense. Science 2006; 313:68 - 71; http://dx.doi.org/10.1126/science.1128214; PMID: 16741077
- Martín-Hernández AM, Baulcombe DC. Tobacco rattle virus 16-kilodalton protein encodes a suppressor of RNA silencing that allows transient viral entry in meristems. J Virol 2008; 82:4064 - 71; http://dx.doi.org/10.1128/JVI.02438-07; PMID: 18272576
- Yelina NE, Savenkov EI, Solovyev AG, Morozov SY, Valkonen JP. Long-distance movement, virulence, and RNA silencing suppression controlled by a single protein in hordei- and potyviruses: complementary functions between virus families. J Virol 2002; 76:12981 - 91; http://dx.doi.org/10.1128/JVI.76.24.12981-12991.2002; PMID: 12438624
- Voinnet O. Non-cell autonomous RNA silencing. FEBS Lett 2005; 579:5858 - 71; http://dx.doi.org/10.1016/j.febslet.2005.09.039; PMID: 16242131
- Baumberger N, Baulcombe DC. Arabidopsis ARGONAUTE1 is an RNA Slicer that selectively recruits microRNAs and short interfering RNAs. Proc Natl Acad Sci U S A 2005; 102:11928 - 33; http://dx.doi.org/10.1073/pnas.0505461102; PMID: 16081530
- Anandalakshmi R, Pruss GJ, Ge X, Marathe R, Mallory AC, Smith TH, Vance VB. A viral suppressor of gene silencing in plants. Proc Natl Acad Sci U S A 1998; 95:13079 - 84; http://dx.doi.org/10.1073/pnas.95.22.13079; PMID: 9789044
- Semashko MA, Rakitina DV, González I, Canto T, Kalinina NO, Taliansky ME. Movement protein of hordeivirus interacts in vitro and in vivo with coilin, a major structural protein of Cajal bodies. Dokl Biochem Biophys 2012; 442:57 - 60; http://dx.doi.org/10.1134/S1607672912010164; PMID: 22419098
- Taliansky M, Kim SH, Mayo MA, Kalinina NO, Fraser G, McGeachy KD, Barker H. Escape of a plant virus from amplicon-mediated RNA silencing is associated with biotic or abiotic stress. Plant J 2004; 39:194 - 205; http://dx.doi.org/10.1111/j.1365-313X.2004.02120.x; PMID: 15225285
- Gushchin VA, Lukhovitskaya NI, Andreev DE, Wright KM, Taliansky ME, Solovyev AG, Morozov SY, MacFarlane SA. Dynamic localization of two tobamovirus ORF6 proteins involves distinct organellar compartments. J Gen Virol 2013; 94:230 - 40; http://dx.doi.org/10.1099/vir.0.045278-0; PMID: 23052393
- Ryabov EV, Robinson DJ, Taliansky ME. A plant virus-encoded protein facilitates long-distance movement of heterologous viral RNA. Proc Natl Acad Sci U S A 1999; 96:1212 - 7; http://dx.doi.org/10.1073/pnas.96.4.1212; PMID: 9990003
- Ascencio-Ibañez JT, Settlage SB. DNA abrasion onto plants is an effective method for geminivirus infection and virus-induced gene silencing. J Virol Methods 2007; 142:198 - 203; http://dx.doi.org/10.1016/j.jviromet.2007.01.031; PMID: 17337069
- Taliansky M, Aranda M, Garcia-Arenal F. Differential invasion by tobamoviruses of Nicotiana megalosiphon following the hypersensitive response. Phytopathology 1994; 84:812 - 5; http://dx.doi.org/10.1094/Phyto-84-812
