Abstract
The ability of adaptive immune system to protect higher vertebrates from pathogens resides in the ability of B and T cells to express different antigen specific receptors and to respond to different threats by activating distinct differentiation and/or activation pathways. In the past 10 years, the major role of epigenetics in controlling molecular mechanisms responsible for these peculiar features and, more in general, for lymphocyte development has become evident. KRAB-ZFPs is the widest family of mammalian transcriptional repressors, which function through the recruitment of the co-factor KRAB-Associated Protein 1 (KAP1) that in turn engages histone modifiers inducing heterochromatin formation. Although most of the studies on KRAB proteins have been performed in embryonic cells, more recent reports highlighted a relevant role for these proteins also in adult tissues. This article will review the role of KRAB-ZFP and KAP1 in the epigenetic control of mouse and human adaptive immune cells.
Epigenetic Control of the Adaptive Immune System
Two pathways of immune responses against foreign invaders characterize higher vertebrates: the innate and the adaptive immune systems. Innate immunity is the first line defense; it is mainly, but not only, mediated by the myeloid lineage of the hematopoietic compartment and relies on the direct recognition of pathogen associated molecules by specific receptors expressed on all innate immune cells. Upon engagement of their receptors, innate immune cells are able to directly and quickly neutralize the pathogen by different means. Adaptive immunity is the second line of defense; it is mainly mediated by the lymphoid (T and B) lineage of the hematopoietic compartment and relies on the recognition of pathogen-associated antigens by specific receptors (T Cell Receptor, TCR, and B cell Receptor, BCR) expressed on adaptive immune cells (each one having a different antigen specificity). Upon engagement of these receptors, the few antigen-specific cells can expand and amplify the signal by activating a cascade of subsequent events, which eventually eliminate the pathogen.
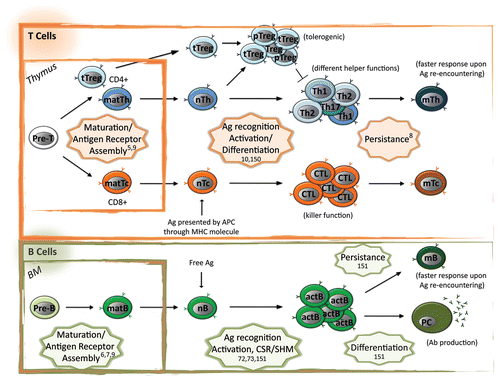
Adaptive immune cells are highly specialized cells characterized by (1) the ability to distinguish a danger (i.e., pathogen antigens) from a false alarm (i.e., self or food antigens) and react accordingly (response or anergy and/or tolerance), (2) a very high level of response/differentiation plasticity (i.e., different cell differentiation in response to different threats), and (3) the ability to persist after clearance of infection and respond faster in case of re-encountering of the same antigen (immunological memory) (see for a schematic of adaptive cell differentiation and function). As a further peculiarity, together with the strict and temporal control of gene expression needed by all differentiation pathways, adaptive immune cell differentiation relies on the unique event of genomic DNA rearrangement at the antigen receptor loci in order to ensure maturation of clones with different antigen specificity. These features of adaptive immune cells are ensured at the molecular level by the cross talk between external stimuli and intrinsic cues during development and activation. In the recent years, epigenetics has been proposed as main hub for the integration of these signals.
Figure 1. Epigenetic control of adaptive immune cell differentiation and function. Adaptive immune system consists in the cellular (T cell mediated) and humoral (B cell mediated) arm. Differentiation of adaptive immune cells occurs initially in primary lymphoid organs (thymus for T cells and bone marrow, BM, for B cells), where each progenitor cell (Pre-T and Pre-B) rearranges its antigen receptor loci at the DNA level in order to express a different receptor. In these organs only cells expressing a functional receptor not recognizing self-antigens receive anti-apoptotic stimuli (positive selection) and survive from negative selection (depletion of autoreactive clones). Upon rearrangement and differentiation effector and regulatory mature cells (matT helper, matTh, or matT cytoxic, matTc, if expressing CD4 or CD8 as TCR co-receptor, respectively, matB and thymic derived CD4+ T regulatory, tTreg) migrate to secondary lymphoid organs (mainly spleen and lymphnodes), where naïve (nTh, nTc, nB, and tTreg) cells can encounter an antigen (Ag). Ag-TCR or Ag-BCR interaction induces pattern of expressions leading to the generation of activated (and thus functional) immune cells. TCR recognizes Ag only if processed and presented by antigen presenting cells (APC) through Major Histocompatibility Class (MHC) molecules, while BCR recognizes free Ag. Depending on the type of Ag and/or the MHC presenting it, naïve cells differentiate in different subsets with different functions: (1) nTh differentiate mainly in Th1, Th2, Th17 cells that produce different cytokines inducing different B cell- mediated or myeloid response to extracellular pathogens; (2) in case of non-harmful Ag, nTh differentiate into T regulatory cells (peripheral, pTreg) that, together with tTreg cells, induce tolerance and dampen immune response through different mechanisms; (3) nTc differentiate into Cytotoxic T Lymphocyte (CTL) that mediate killing of the infected target cells; (4) nB cells differentiate into activated B (actB) cells and this process requires antibody maturation and differentiation through class switch recombination (CSR) and somatic hyper mutation (SHM), respectively; actB cells further differentiate in plasma cells (PC) that are factories for the secretion of antibodies (Ab). Most of the expanded activated cells undergo apoptosis after pathogen clearance while a small fraction persists in the body as memory cells (mTh, mTc, and mB) and mediate faster secondary response in case of re-encountering of the Ag. The adaptive immune cell processes controlled at the epigenetic level are highlighted in stars with relative references.
Epigenetics collectively defines inheritable post-translational modifications of the chromatin components (DNA and histones) that are not directly dictated by the underlying DNA sequence. These modifications result in changes in the compaction status and nuclear localization of chromatin, and ultimately govern gene expression patterns. Histone acetylation is usually associated with transcription permissive chromatin, with acetylated lysine 9 and lysine 27 on histone 3 (H3K9Ac and H3K27Ac, respectively) among the best characterized permissive marks. With some exceptions, methylated histones are instead associated with non-permissive chromatin, such as H3K9me and H3K27me.Citation1-Citation3
Control of T and B cell lineage specification has been originally ascribed to the activity of the so-called lineage-determining factors (i.e., transcription factors whose expression is sufficient and necessary for the determination of a given cell fate). Recently, the activity of these factors has been coupled to chromatin modifiers, and epigenetic modifications have been shown to play key roles in the control of hematopoietic cell differentiation and function.Citation4 In particular, T cell differentiation in the thymus has been linked to progressive chromatin condensation and shown to be controlled by the differential expression of master transcription factors recruiting histone deacetylases, acetyl transferases, methyltransferases, and components of the Polycomb and NuRD complexes at specific genomic loci.Citation5 In a very similar manner, B cell fate is determined by the interaction of key transcription factors with epigenetic modifiers that alters the epigenetic landscape and regulates the gene expression pattern of the differentiating cells.Citation6,Citation7 Furthermore, the specific abilities of adaptive immune cells of rearranging DNA at the antigen receptor loci and “remembering” a stimulus (memory) are strictly controlled at the epigenetic level. Antigen receptor rearrangement (DNA recombination of DNA stretches encoding common and variable domains of the receptor) relies on both chromatin modifiers for the recruitment and activation of the recombinase enzymes, and chromatin conformation and nuclear structure to put genetic elements distributed along large genomic distances in proximity.Citation8,Citation9 These data strongly supported the idea that lymphoid cell differentiation is governed by the ability of master transcription factors and chromatin modifying enzymes to induce activation of lineage specific genes and repression of alternative lineage-related ones. As occurring in several differentiation pathways, this might lead to an irreversible epigenetic landscape with mutually exclusive epigenetic modifications at the lineage-determining transcription factor loci (i.e., open chromatin conformation at the specific lineage factor and closed conformation at the alternative-lineage factors).Citation10 Nevertheless, the development of high-throughput sequencing techniques has, at least partially, disproved this scenario. By assessing genome-wide chromatin modifications and gene expression, chromatin landscape in mature T and B cell subsets has been found far more dynamic than previously expected, showing “bivalent” states (i.e., loci marked by open and closed chromatin marks together) and fast changes in epigenetic modifications in response to external stimuli both in human and mouse cells.Citation11-Citation13
As expected from the described role of epigenetics in regulating virtually all the aspects of the adaptive immune system, defects in chromatin modifiers and aberrant epigenetic control have been linked to human immune-related diseases. In particular, alteration in epigenetic regulation seems to concur with genetic predisposition in the pathogenesis of autoimmune disorders, such as systemic lupus erythematosus, rheumatoid arthritis, systemic sclerosis, and type 1 diabetes.Citation14 Studies performed in monozygotic twins non-concordant for the development of the disease showed global DNA and/or histone hypomethylation together with locus specific DNA hypomethylation of genes associated to activation and/or response of adaptive immune cells in affected patients.Citation14 Similarly, altered epigenetic regulation at key immune genes during development has been proposed as functional link between environmental exposures and chronic inflammation leading to allergies.Citation15 Although the molecular mechanisms underlying this observation are still unclear, several mutations in chromatin modifying molecule expressing genes have been also associated to leukemia development.Citation16
Overall, all of these studies assessed the pivotal role of epigenetics in controlling most of the molecular mechanisms that regulate adaptive immune cell features, including their peculiar high level of plasticity.
KRAB-ZFP and KAP1
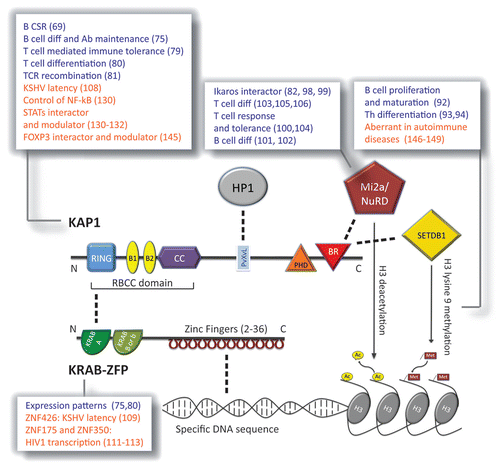
Krüppel-associated box zinc finger proteins (KRAB-ZFPs or ZNFs) constitute the widest family of tetrapod-specific transcription repressors, which underwent a marked expansion by gene and segment duplication during evolution.Citation17-Citation20 KRAB-ZFPs are characterized by tandem repeats of C2H2 zinc fingers at the C-terminus, which confer them with the ability to bind specific polynucleotidic sequences, and one or two KRAB domains at the N-terminus, responsible for recruiting KRAB-associated protein 1 (KAP1) ().Citation21-Citation23 KAP1 is the so far only described co-factor of KRAB-ZFPs, is a ubiquitously expressed member of the tripartite motif-containing (TRIM) family, and is also known as TRIM28, TIF1β, or KRIP1. It encodes a TRIM/RBCC motif (RING finger, B box, coiled coil), plant homeodomain finger and bromodomain and functions as a strong transcriptional repressor when bound to DNA.Citation24-Citation26 It acts as scaffold that recruits chromatin modifiers including the SETDB1 histone methyltransferase, the CHD3/Mi2 component of the NuRD complex and Heterochromatin Protein 1 (HP1). These KAP1-mediated complex leads to heterochromatin formation by histone 3 tri-methylation on lysine 9 (H3K9me3) and histone deacetylation ().Citation27-Citation29 The rather advanced characterization of the biochemical mechanism of KRAB-ZFP/KAP1 action contrasts with our large ignorance of the physiological roles of this system, in particular in adult tissues. KRAB-ZFP genes are evolutively recent and their expansion in the tetrapod genome strongly suggests that new functions for the encoded proteins have been generated under the selective pressure of newly acquired biological pathways. It is tempting to speculate that one of these pathways may well be the adaptive immune system, as typical instance of acquisition of new specialized functions in higher vertebrates.
Figure 2. Function(s) of the KRAB proteins. Schematic of the main domains of KAP1 (top) and a prototypic KRAB-ZFP (bottom), and of the main molecules interacting with each of the two (direct interaction is depicted as dashed line). KAP1 domains (N to C-terminal). RING, Really Interesting New Gene zinc finger; B1 and B2, B box zinc fingers; CC, coiled coil. RING, B boxes, and CC form together the RBCC domain, needed by KAP1 to interact with the KRAB A domain of the KRAB-ZFP. PvXvL, hydrophobic pentapeptide needed for interaction with HP1 (Heterochromatin Protein 1); PHD, plant homeo-domain; BR, bromodomain. PHD and BR cooperate in binding the Mi2a/NuRD deacetylase complex and the SETDB1 histone 3 lysine 9 methyl transferase. Once recruited by KAP1 these two histone-modifying factors are able to induce heterochromatin formation by modifying histone 3 (H3) tail. KRAB-ZFP domains (N to C-terminal). KRAB A, Krüppel-associated box A. It is a transcriptional repressor module present in all KRAB-ZFP and mediates KAP1 recruitment. The second KRAB box is facultative and can be B or b depending on the primary structure of the sequence. Zinc fingers are present in tandem repeats and can vary from 2 to 36 in the family; they are able to bind specific DNA sequences. KAP1 has not a DNA-binding domain; KRAB-ZFP is thus the linkage between specific DNA stretches and KAP1-mediated complexes. Listed in the pop-ups the roles of the depicted protein or histone modification as assessed in the mouse (in blue) and in the human (in orange) adaptive immune system; in brackets the relative references.
Several studies on the KRAB-ZFP/KAP1 system have been focused on the mouse embryo and embryonic stem cell (ESC) biology. They have shown an essential role for KAP1 in embryonic differentiation and morphogenesis, establishment and/or maintenance of genomic imprinting and pluripotency and/or self-renewal maintenance in embryonic stem cells, where it also represses endogenous and exogenous retroviruses.Citation30-Citation37 The KRAB-ZFPs mediating KAP1 action in these processes have been identified as zfp809, zfp568 (chato), and zfp57 for the control of exogenous retroviruses, extra-embryonic tissue development, and genomic imprinting, respectively.Citation38-Citation41 Zfp819 has been proposed very recently as player in the control of endogenous retroelement in ESC.Citation42 Also gametogenesis seems to be controlled by the KRAB-ZFP/KAP1 system, with KAP1 needed for spermatogenesis and KRAB ZNF899 (Prdm9) playing a key role in meiotic recombination and hybrid sterility.Citation43-Citation46
In human adult tissues, consistent studies have demonstrated the role of the KRAB-ZFP/KAP1 system in vital cellular pathways.Citation47 They have found that during DNA damage response, Ataxia Telangiectasia Mutated- (ATM-) mediated KAP1 phosphorilation is required for chromatin relaxation and recruitment of the DNA repairing complex at the damage site.Citation48-Citation50 Also, KRAB-ZFP/KAP1 system plays a double role in neoplastic transformation. On one side, the KRAB ZNF350 (ZBRK1) partakes in human cancer development by regulating the expression of oncogene and/or oncosuppressor genes and mediating the DNA damage response controlled by the tumor-suppressor molecule retinoblastoma.Citation51-Citation54 On the other, KAP1 controls p21 and p53 pathways by different means and its overexpression is associated with several human malignancies.Citation55-Citation58 Moreover, p53 activity seems to be directly regulated by the KRAB-ZFP ZNF420 (APAK).Citation59,Citation60 A very recent study has also proposed KRAB ZFP1 as negative regulator of polymerase II-mediated transcription.Citation61
Regarding the physiology of specific tissue, KAP1 and ZNF746 (PARIS) have been implicated in central nervous system disorders in mice and humans, respectively,Citation62,Citation63 while KAP1 and the KRAB-ZFP rsl1-2 seem to control mouse liver metabolism and sexual dimorphism, respectively.Citation64,Citation65 KRAB zfp157 has been proposed as player in controlling proper mammary gland development in the mouse.Citation66 Very recently, a prominent role of KAP1 in controlling microRNA expression regulating mouse erythroid differentiation has also been described.Citation67
KAP1/KRAB-ZFP in Mouse Lymphoid Cell Development
The role of KAP1 and KRAB-ZFPs in the mouse and human adaptive immune systems is far from being clearly assessed. Nevertheless, the study of mouse models conditionally knocked out for the KAP1 gene or genes encoding for its interacting partners has put forth a relevant role for this system in several aspects of the adaptive immune response.
B cell lineage specific Kap1 KO mice have been developed by crossing the CD19Cre/+, in which the recombinase is expressed at the pro-B stage, with the kap1fl/fl strain.Citation30,Citation68 A first study by Reina-San Martin group has found an interaction between kap1 and Activation-Induced cytidine Deaminase (AID) in mouse B cells and used cells from KO mice to study the molecular relevance of this interaction.Citation69 AID is the enzyme required for initiating class switch recombination (CSR) and somatic hypermutation (SHM) in B cells.Citation70,Citation71 SHM and CSR are two processes needed for antibody maturation and diversification in activated B cells, and are based on the introduction of point mutations and recombination in the immunoglobulin gene, respectively.Citation72,Citation73 The epigenetic machinery strictly regulates these pathways by controlling accessibility of the loci to AID, three-dimensional localization of the recombining regions and recruitment of the DNA damage response machinery.Citation74 In the above-mentioned study, the authors showed a 50% reduction in CSR in kap1 KO when compared with control B cells in vitro and no defect in SHM. They proposed a role for kap1 in facilitating recruitment of AID to the switch region through the formation of a complex containing HP1 and recognizing H3K9me3.Citation69 By analyzing the same mouse model, we have found normal rates of CSR in vivo, probably because of compensating mechanisms masking in vivo the mild defect observed in vitro.Citation75 Instead, we have observed a significant defect in mature B cell development, in particular in the non-conventional subset, decreased levels of steady-state antibody production, and faster rates of antibody decays after viral immunization. This indicated a relevant role for KAP1 in B cell differentiation and maturation. By performing gene expression and chromatin precipitation studies, we found the PI3K antagonist PTEN to be directly regulated by kap1 and proposed altered regulation of this gene—known to be a main player in B cell biologyCitation76—as molecular mechanism underlying (at least part of) the phenotypes observed in kap1 KO mice. Genome wide, we found that kap1 binding sites correlated positively with regions marked by the repressive histone modification H3K9me3 and negatively with B cell-specific regulatory elements bearing active marks (H3K4me1 and H3K4me3) or bound by the PU.1 transcription factor.Citation75,Citation77,Citation78 Kap1 binding sites seemed also to be associated to facultative rather than constitutive heterochromatin.Citation75
Three studies have investigated the function of kap1 in mouse T cells. By using conditional KO mice based on the expression of the cre recombinase under the T cell co-receptor CD4 or the T cell specific kinase lck promoter, we and others have reported kap1 involvement in controlling T cell-mediated response and tolerance.Citation79,Citation80 The two studies performed gene expression analyses and proposed different molecular mechanisms as origin of the observed defects. Tasuku Honjo’s group proposed that kap1 regulated transforming growth factor pathway in mature T cells and that alteration of this axis led to autoimmune phenotype.Citation79 We found profound defects in immature T cell differentiation and proposed kap1-mediated direct control of the FoxO1 gene—encoding for the major regulator of thymocyte transcriptional network—as the main pathway underlying this phenotype.Citation80 The same year, another group confirmed the role of kap1 in T cell differentiation and extended it to invariant natural killer T cells, but attributed the phenotype to a role for this molecule in TCR rearrangement.Citation81 We observed association between kap1, NuRD, and Ikaros binding sites, as expected.Citation28,Citation82 Nevertheless, our chromatin studies showed an unpredicted landscape for kap1 binding sites in immature T cells. As for B cells, kap1 binding sites were highly enriched in repressive (H3K9me3 and H3K27me3) and depleted of active promoters and/or enhancer associated (H3K4me3, H3K4me1, and H3K9ac) histone marks. Differently from what observed in mature B cells and unexpected for a predicted transcriptional repressor, markers of open chromatin and/or active transcription, such as the histone acetyl-transferase CBP, ETS1 (a critical transcriptional regulator of T cells), TFIIB, and formaldehyde-assisted isolation of regulatory elements (FAIRE), were significantly enriched at kap1 binding sites. Although we could not rule out the possibility of retrieving different complexes containing kap1 in different cells, these data suggested that kap1 binds plastic cis-regulatory regions and may coexist at these sequences with partially assembled and/or weakly bound complexes of transcription and chromatin-remodelling factors in immature T cells. In order to assess if the different features of kap1 binding sites in T and B cells are linked to the different lineages or to the diverse differentiation stages (immature and mature, respectively), further investigation is needed. In line with the hypothesis of the formation of different KRAB complexes in different cellular contests, in non-lymphoid cells several studies have suggested that KRAB proteins may play different functions and even acquire activating transcriptional ability depending on the interacting partners.Citation83-Citation86
Functional studies about KRAB-ZFPs in the mouse lymphoid system are not available. The only existing data are related to their level of expression in different hematopoietic lineages. Data from ChiP-seq studies also indicated that kap1 binds in proximity of KRAB-ZFP genes in mouse adaptive immune cells,Citation75,Citation80 suggesting that it could be involved in the regulation of expression or maintenance of genomic stability of these highly repeated sequences as proposed for human cells.Citation87-Citation91
Further hints about the functional role of kap1 in adaptive immune cell development might be extrapolated from studies performed on kap1-interacting molecules or associated chromatin modifications. In vitro and in vivo data have demonstrated kap1 association with the histone deacetylases NuRD complex, SETDB1 histone methyltransferase, HP1, and H3K9me3.Citation27-Citation29,Citation75,Citation80,Citation87
The role of H3K9me in lymphoid lineage development has been studied by taking advantage of mouse models knockout for the H3K9 methyltransferases (SUV39H1, G9a, and SETDB1). In the first report showing G9a KO mice, no major phenotype in the T cell compartment was observed (T helper specific functions were not assessed). Interestingly, B cell phenotype seemed fully consistent with the one observed in kap1 KO mice, as B cells showed a lower proliferation capacity and defects in rearrangement of the Ig locus and plasma cell maturation.Citation92 Two studies showed the importance of H3K9me in T helper differentiation and function. By using G9a KO CD4+ cells, Zaph’s group proposed a role for H3K9me2 in controlling interleukin 17 gene locus and T helper function.Citation93 A few years later, Amigorena’s group used Suv39h1 and HP1 KO CD4+ cells and showed SUV39H1-HP1-H3K9me3 controlling interferon γ and interleukin 4 gene loci and thus T helper differentiation and stability.Citation94 All of these findings suggested that kap1 and H3K9me affect a quite restricted set of processes during lymphocyte development and activation, and this was rather unexpected for molecules with such a broad distribution in the genome.Citation75,Citation80 Nevertheless, this behavior has been attributed to several histone-modifying factors, such as the MLL/SET H3K4 and the Polycomb H3K27 methyltransferase complexes and the H3K27 demethylase Jmjd3.Citation95-Citation97 Thus, in analogy with other transcriptional co-regulators, kap1 and H3K9me3 may fine-tune expression rather than acting like an on-off switch of gene expression, at least in the lymphoid system.
A more pronounced role in lymphoid cell physiology seems to be played by the NuRD histone deacetylase complex, whose activity in this compartment seems to be mainly dictated by its interaction with the zinc finger Ikaros, a key regulator of lymphopoiesis.Citation82,Citation98,Citation99 Different reports have shown that NuRD and/or the Ikaros-NuRD complex regulates key pathways of lymphoid cell biology, including (1) CD8 and CD4 gene expression and T cell development, (2) cytokine loci and T cell immune response, tolerance and anergy, and (3) B cell specific genes and B cell differentiation.Citation100-Citation106 Direct evidence of binding of kap1 to Ikaros/NuRD in lymphoid cells is still missing. Nevertheless, in vitro interaction with NuRD and in vivo overlapping of kap1 and Ikaros-NuRD binding sites might lead to contemplate kap1 involvement in the control of, at least some, Ikaros-NuRD target genes. Moreover, these data might suggest that kap1 can be recruited to target genes through interaction with other DNA binding factors and independently by KRAB-ZFP mechanisms, as also proposed in other contexts (see also following paragraph).Citation88
KAP1/KRAB-ZFP in the Human Immune System
Direct and indirect evidences link KRAB proteins to different aspects of human adaptive immune cell differentiation and function.
The best-characterized function of KRAB-ZFP/KAP1 system in human adaptive immune cells is the control of viral replication. Apparently contrasting results have been published about the role of this system in the control of B cell tropic herpes viruses. These viruses are characterized by two different stages of life cycle (the latent/silent and the lytic/active) that are strictly regulated by activation and/or repression of specific viral gene expression patterns. On one side, ZNF251 (KZLP) has been shown to activate K1 promoter of Kaposi’s sarcoma-associated herpes virus (KSHV) and contrast the LANA KSHV, which is the essential transcription factor to establish latency.Citation107 Nevertheless, this early report, which was mostly based on biochemical data, did not show binding of the ZFP to viral DNA and did not show a role for this ZFP in the replication of KSHV DNA. Thus, the relevance of this study is not obvious. More consistently, KRAB-ZFP and KAP1 have been linked to repression of KSHV activation and induction of latency. Although there is no evidence for a direct interaction between the two KRAB proteins in this context, ZNF426 (K-RBP) has been shown to directly repress transcription of the KSHV transactivator RTA gene and KAP1 to bind and repress several KSHV lytic genes.Citation108,Citation109 KAP1 and the KRAB-ZFP ZBRK1 have also been shown to interact with Epstein-Barr herpes virus (EBV), although the role of this complex on EBV life cycle has not been clearly defined.Citation110 Moreover, KRAB-ZFP/KAP1 are apparently involved in the control of Human Immunodeficiency Virus (HIV) replication at different stages. HIV targets CD4+ (T and monocyte) cells and is characterized by the ability of integrate into the human genome and establish in some, not fully elucidated, circumstances latent infection. Two KRAB-ZFPs have been proposed to regulate HIV transcription. Early studies have identified ZNF175 (OTK18) as gene upregulated upon HIV infection in monocyte-derived macrophages and shown its role in controlling several cellular genes as well as HIV transcription.Citation111,Citation112 A more recent study has reported ZBRK1 to bind and control HIV promoter expression by recruiting KAP1.Citation113 In this work, the authors have also shown that knocking down of ZBRK1 reduced KAP1 binding to HIV promoter by only 25%, supporting the idea that KAP1 binding to HIV sequences might be mediated by more than one (KRAB) factor. Another group has recently put forward a role for KAP1 in controlling HIV integration into the cell host genome.Citation114 This study reported increased integrated viral copies upon KAP1 downregulation and proposed a role in post-translational modification of the HIV integrase protein, rather than epigenetic for KAP1. All of these observations suggest that KRAB proteins conserved the antiviral activity (mainly mediated by the control of expression of viral genes) from mouse to human, although by using different mechanisms in the two systems. Further investigating this issue in human adaptive immune cells might lead to the discovery of new pathways underlying the still obscure process of epigenetic control of viral latency and ameliorate both basic scientific knowledge and antiviral therapies.
As aforementioned, in order for the DNA damage response (DDR) machinery to be recruited at the DNA break point, KAP1 needs to be phosphorylated by ATM to detach and allow chromatin relaxation.Citation48-Citation50 The DDR pathway is required for proper DNA rearrangement at the TCR and BCR loci and for CSR/SHM at the immunoglobulin locus in B cells. Indeed, although the recombinase proteins acting at the receptor loci are specific for the immature T and B cells, the machinery recruited to the DNA break induced by the recombinases is the canonical DDR. DDR is mediated by the non-homologous end-joining apparatus, which includes the DNA-dependent protein kinase catalytic subunit (DNA-PKcs) and ATM in this context.Citation115 Although neither ATM nor DNA-PK are absolutely required, their combined deficiency results in a block in DNA recombination suggesting that these two kinases have overlapping activities.Citation116,Citation117 The important role of this machinery in the assembly of antigen receptor genes is highlighted by the observation that mutations in DNA-PK leads to severe combined immune deficiency (SCID), while ATM deficiency causes ataxia-telangiectasia (A-T) disease in humans.Citation118-Citation120 A-T patients show severe neurodegenerative disorder usually associated with immunological dysfunctions, such as decreased specific immunoglobulin production and CD4+ T cell number, increased radiation susceptibility and predisposition to lymphoid cancer.Citation121 A-T immunological abnormalities have been associated to defective receptor locus recombination, and T and B cell progenitor maturation in mouse models.Citation122-Citation127 Further investigation will clarify the function of KAP1 in this context confirming or not its relevance put forth by the recent data reporting its prominent role in ATM mediated response.
The activity of transcription factors playing pivotal roles in human lymphocyte biology has also been linked to KRAB proteins. c-Rel/NFkB is a family of transcription factors regulating immune response and inflammation through their control of immune genes, such as interleukins and lineage specific transcription factors.Citation128 Upon nuclear translocation NFkB transcriptional activity is controlled by post-translational modifications that regulate its nuclear retention and interaction with co-activators or repressors.Citation129 KAP1 has been proposed as negative regulator of NFkB transcriptional activity by interacting with the acetyltransferase p300/CBP and inhibiting acetylation of NFkB at the interleukin-6 inflammatory gene.Citation130 This KAP1 activity seemed to be mediated by its interaction with the signal transducers and activators of transcription 3 (STAT3).Citation131 KAP1 seems also to play a role in the interferon-mediated inflammatory response by interacting with STAT1.Citation132 These data are particularly interesting in light of the importance of STAT proteins in shaping immune response. STATs are indeed main activators of cytokine genes involved in T helper (Th), T cytotoxic, and B cell differentiation and/or activation and exert their positive function by recruiting the histone acetyltranferase p300/CBP.Citation133-Citation135 In light of this, if confirmed, KAP1 interaction and/or regulation of STAT proteins might imply a major role for this protein in the regulation of gene expression patterns of human adaptive immune cells. KAP1 interaction with STAT3 seems particularly interesting in light of the role of the latter in controlling autoimmune manifestations. It has been clearly demonstrated, indeed, the main role of STAT3 in both human and mouse in inducing the expression of cytokine genes leading to Th17 phenotype, which is the main Th subset involved in inflammation during autoimmune manifestations.Citation136-Citation138 On the other hand, there seems to be discordance, at least in the mouse, about the role of STAT3 in controlling the pattern of expression in Treg cells, which is the T subset counteracting autoimmune manifestations by dampening immune response through different mechanisms.Citation139,Citation140 The master transcription factor regulating Treg cell specification is FOXP3, whose gene mutation induces a life-threatening autoimmune disorder called IPEX.Citation141,Citation142 This forkhead-domain factor acts as either activator or repressor of gene expression thanks to its ability to bind gene promoters and cooperate with different component of the epigenetic machinery.Citation143 Among several other interactors, recent data indicated that FOXP3 associates with STAT3Citation144 and KAP1Citation145 in human Treg cells. FOXP3-KAP1 interaction seemed to be mediated by a human specific KRAB-containing protein derived from the alternative splicing of the ZFP90 and called FIK, and to be necessary for the repressive activity of FOXP3 at target gene loci. Thus, according to these reports and mirroring the mouse system, KAP1 seems to be a player in the maintenance of human immune tolerance through different means. This role of KAP1 in controlling autoimmunity seems highly consistent with the altered H3K9me pattern and HP1 recruitment at key immune genes found in the lymphocytes of patients suffering from several autoimmune diseases.Citation146-Citation149
Concluding Remarks
Although not many reports have studied the role of KRAB proteins in controlling gene expression patterns in immune cells, several lines of evidence both in the mouse and in humans put forth a relevant role for these proteins in key aspects of the biology of these peculiar cells. Interestingly, KRAB proteins seem to form different complexes depending on the cellular contest (i.e., level of expression of common and cell type-specific transcription factors, post-translational modification of transcription, and chromatin regulators) and this seems to significantly affect the function of these proteins and the resulting chromatin modifications. More focused studies, including high throughput gene expression and chromatin approaches, will clarify the molecular patterns mediated by KRAB proteins and improve the understanding of the complicate epigenetic landscape controlling adaptive immune cell differentiation and function.
| Abbreviations: | ||
| AID | = | Activation-Induced cytidine Deaminase |
| A-T | = | Ataxia-Telangectasia |
| ATM | = | Ataxia-Telangectasia Mutated |
| BCR | = | B Cell Receptor |
| CSR | = | Class Switch Recombination |
| DDR | = | DNA Damage Response |
| EBV | = | Epstein-Barr Virus |
| ESC | = | Embryonic Stem Cell |
| H3K9me3 | = | Histone 3 Lysine 9 three-methylation |
| HIV | = | Human Immunodeficiency Virus |
| HP1 | = | Heterochromatin Protein 1 |
| KAP1 | = | KRAB-Associated Protein 1 |
| KRAB | = | Kruppel Associated Box |
| KSHV | = | Kaposi Sarcoma associated Herpes Virus |
| NuRD | = | Nucleosome Remodeling Deacetylase |
| SCID | = | Severe Combined Immune Deficiency |
| SETDB1 | = | SET Domain, Bifurcated 1 |
| SHM | = | Somatic HyperMutation |
| TCR | = | T Cell Receptor |
| Th | = | T helper |
| ZNF | = | Zinc Finger |
| ZFP | = | Zinc Finger Protein |
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Acknowledgments
Thanks to Didier Trono, Monica Ievolella, Laura Passerini, and Rosa Bacchetta for fruitful discussions. F.R.S.d.S is supported by the Italian Ministry of Health (Grant GR-2010-2317550).
References
- Jenuwein T, Allis CD. Translating the histone code. Science 2001; 293:1074 - 80; http://dx.doi.org/10.1126/science.1063127; PMID: 11498575
- Bernstein BE, Meissner A, Lander ES. The mammalian epigenome. Cell 2007; 128:669 - 81; http://dx.doi.org/10.1016/j.cell.2007.01.033; PMID: 17320505
- Goldberg AD, Allis CD, Bernstein E. Epigenetics: a landscape takes shape. Cell 2007; 128:635 - 8; http://dx.doi.org/10.1016/j.cell.2007.02.006; PMID: 17320500
- Heinz S, Glass CK. Roles of lineage-determining transcription factors in establishing open chromatin: lessons from high-throughput studies. Curr Top Microbiol Immunol 2012; 356:1 - 15; http://dx.doi.org/10.1007/82_2011_142; PMID: 21744305
- Taniuchi I, Ellmeier W. Transcriptional and epigenetic regulation of CD4/CD8 lineage choice. Adv Immunol 2011; 110:71 - 110; http://dx.doi.org/10.1016/B978-0-12-387663-8.00003-X; PMID: 21762816
- Hagman J, Ramírez J, Lukin K. B lymphocyte lineage specification, commitment and epigenetic control of transcription by early B cell factor 1. Curr Top Microbiol Immunol 2012; 356:17 - 38; http://dx.doi.org/10.1007/82_2011_139; PMID: 21735360
- Medvedovic J, Ebert A, Tagoh H, Busslinger M. Pax5: a master regulator of B cell development and leukemogenesis. Adv Immunol 2011; 111:179 - 206; http://dx.doi.org/10.1016/B978-0-12-385991-4.00005-2; PMID: 21970955
- Weng NP, Araki Y, Subedi K. The molecular basis of the memory T cell response: differential gene expression and its epigenetic regulation. Nat Rev Immunol 2012; 12:306 - 15; http://dx.doi.org/10.1038/nri3173; PMID: 22421787
- Bossen C, Mansson R, Murre C. Chromatin topology and the regulation of antigen receptor assembly. Annu Rev Immunol 2012; 30:337 - 56; http://dx.doi.org/10.1146/annurev-immunol-020711-075003; PMID: 22224771
- Wilson CB, Rowell E, Sekimata M. Epigenetic control of T-helper-cell differentiation. Nat Rev Immunol 2009; 9:91 - 105; http://dx.doi.org/10.1038/nri2487; PMID: 19151746
- Wei G, Wei L, Zhu J, Zang C, Hu-Li J, Yao Z, Cui K, Kanno Y, Roh TY, Watford WT, et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity 2009; 30:155 - 67; http://dx.doi.org/10.1016/j.immuni.2008.12.009; PMID: 19144320
- Schmidl C, Klug M, Boeld TJ, Andreesen R, Hoffmann P, Edinger M, Rehli M. Lineage-specific DNA methylation in T cells correlates with histone methylation and enhancer activity. Genome Res 2009; 19:1165 - 74; http://dx.doi.org/10.1101/gr.091470.109; PMID: 19494038
- Abraham BJ, Cui K, Tang Q, Zhao K. Dynamic regulation of epigenomic landscapes during hematopoiesis. BMC Genomics 2013; 14:193; http://dx.doi.org/10.1186/1471-2164-14-193; PMID: 23510235
- Lu Q. The critical importance of epigenetics in autoimmunity. J Autoimmun 2013; 41:1 - 5; http://dx.doi.org/10.1016/j.jaut.2013.01.010; PMID: 23375849
- Martino DJ, Prescott SL. Progress in understanding the epigenetic basis for immune development, immune function, and the rising incidence of allergic disease. Curr Allergy Asthma Rep 2013; 13:85 - 92; http://dx.doi.org/10.1007/s11882-012-0312-1; PMID: 23054626
- Ntziachristos P, Mullenders J, Trimarchi T, Aifantis I. Mechanisms of epigenetic regulation of leukemia onset and progression. Adv Immunol 2013; 117:1 - 38; http://dx.doi.org/10.1016/B978-0-12-410524-9.00001-3; PMID: 23611284
- Emerson RO, Thomas JH. Adaptive evolution in zinc finger transcription factors. PLoS Genet 2009; 5:e1000325; http://dx.doi.org/10.1371/journal.pgen.1000325; PMID: 19119423
- Huntley S, Baggott DM, Hamilton AT, Tran-Gyamfi M, Yang S, Kim J, Gordon L, Branscomb E, Stubbs L. A comprehensive catalog of human KRAB-associated zinc finger genes: insights into the evolutionary history of a large family of transcriptional repressors. Genome Res 2006; 16:669 - 77; http://dx.doi.org/10.1101/gr.4842106; PMID: 16606702
- Looman C, Abrink M, Mark C, Hellman L. KRAB zinc finger proteins: an analysis of the molecular mechanisms governing their increase in numbers and complexity during evolution. Mol Biol Evol 2002; 19:2118 - 30; http://dx.doi.org/10.1093/oxfordjournals.molbev.a004037; PMID: 12446804
- Thomas JH, Emerson RO. Evolution of C2H2-zinc finger genes revisited. BMC Evol Biol 2009; 9:51; http://dx.doi.org/10.1186/1471-2148-9-51; PMID: 19261184
- Peng H, Begg GE, Harper SL, Friedman JR, Speicher DW, Rauscher FJ 3rd. Biochemical analysis of the Kruppel-associated box (KRAB) transcriptional repression domain. J Biol Chem 2000; 275:18000 - 10; http://dx.doi.org/10.1074/jbc.M001499200; PMID: 10748030
- Peng H, Feldman I, Rauscher FJ 3rd. Hetero-oligomerization among the TIF family of RBCC/TRIM domain-containing nuclear cofactors: a potential mechanism for regulating the switch between coactivation and corepression. J Mol Biol 2002; 320:629 - 44; http://dx.doi.org/10.1016/S0022-2836(02)00477-1; PMID: 12096914
- Urrutia R. KRAB-containing zinc-finger repressor proteins. Genome Biol 2003; 4:231; http://dx.doi.org/10.1186/gb-2003-4-10-231; PMID: 14519192
- Friedman JR, Fredericks WJ, Jensen DE, Speicher DW, Huang XP, Neilson EG, Rauscher FJ 3rd. KAP-1, a novel corepressor for the highly conserved KRAB repression domain. Genes Dev 1996; 10:2067 - 78; http://dx.doi.org/10.1101/gad.10.16.2067; PMID: 8769649
- Moosmann P, Georgiev O, Le Douarin B, Bourquin JP, Schaffner W. Transcriptional repression by RING finger protein TIF1 beta that interacts with the KRAB repressor domain of KOX1. Nucleic Acids Res 1996; 24:4859 - 67; http://dx.doi.org/10.1093/nar/24.24.4859; PMID: 9016654
- Kim SS, Chen YM, O’Leary E, Witzgall R, Vidal M, Bonventre JV. A novel member of the RING finger family, KRIP-1, associates with the KRAB-A transcriptional repressor domain of zinc finger proteins. Proc Natl Acad Sci U S A 1996; 93:15299 - 304; http://dx.doi.org/10.1073/pnas.93.26.15299; PMID: 8986806
- Sripathy SP, Stevens J, Schultz DC. The KAP1 corepressor functions to coordinate the assembly of de novo HP1-demarcated microenvironments of heterochromatin required for KRAB zinc finger protein-mediated transcriptional repression. Mol Cell Biol 2006; 26:8623 - 38; http://dx.doi.org/10.1128/MCB.00487-06; PMID: 16954381
- Schultz DC, Friedman JR, Rauscher FJ 3rd. Targeting histone deacetylase complexes via KRAB-zinc finger proteins: the PHD and bromodomains of KAP-1 form a cooperative unit that recruits a novel isoform of the Mi-2alpha subunit of NuRD. Genes Dev 2001; 15:428 - 43; http://dx.doi.org/10.1101/gad.869501; PMID: 11230151
- Schultz DC, Ayyanathan K, Negorev D, Maul GG, Rauscher FJ 3rd. SETDB1: a novel KAP-1-associated histone H3, lysine 9-specific methyltransferase that contributes to HP1-mediated silencing of euchromatic genes by KRAB zinc-finger proteins. Genes Dev 2002; 16:919 - 32; http://dx.doi.org/10.1101/gad.973302; PMID: 11959841
- Cammas F, Mark M, Dollé P, Dierich A, Chambon P, Losson R. Mice lacking the transcriptional corepressor TIF1beta are defective in early postimplantation development. Development 2000; 127:2955 - 63; PMID: 10851139
- Wolf D, Goff SP. TRIM28 mediates primer binding site-targeted silencing of murine leukemia virus in embryonic cells. Cell 2007; 131:46 - 57; http://dx.doi.org/10.1016/j.cell.2007.07.026; PMID: 17923087
- Rowe HM, Jakobsson J, Mesnard D, Rougemont J, Reynard S, Aktas T, Maillard PV, Layard-Liesching H, Verp S, Marquis J, et al. KAP1 controls endogenous retroviruses in embryonic stem cells. Nature 2010; 463:237 - 40; http://dx.doi.org/10.1038/nature08674; PMID: 20075919
- Hu G, Kim J, Xu Q, Leng Y, Orkin SH, Elledge SJ. A genome-wide RNAi screen identifies a new transcriptional module required for self-renewal. Genes Dev 2009; 23:837 - 48; http://dx.doi.org/10.1101/gad.1769609; PMID: 19339689
- Seki Y, Kurisaki A, Watanabe-Susaki K, Nakajima Y, Nakanishi M, Arai Y, Shiota K, Sugino H, Asashima M. TIF1beta regulates the pluripotency of embryonic stem cells in a phosphorylation-dependent manner. Proc Natl Acad Sci U S A 2010; 107:10926 - 31; http://dx.doi.org/10.1073/pnas.0907601107; PMID: 20508149
- Messerschmidt DM, de Vries W, Ito M, Solter D, Ferguson-Smith A, Knowles BB. Trim28 is required for epigenetic stability during mouse oocyte to embryo transition. Science 2012; 335:1499 - 502; http://dx.doi.org/10.1126/science.1216154; PMID: 22442485
- Shibata M, Blauvelt KE, Liem KF Jr., García-García MJ. TRIM28 is required by the mouse KRAB domain protein ZFP568 to control convergent extension and morphogenesis of extra-embryonic tissues. Development 2011; 138:5333 - 43; http://dx.doi.org/10.1242/dev.072546; PMID: 22110054
- Quenneville S, Verde G, Corsinotti A, Kapopoulou A, Jakobsson J, Offner S, Baglivo I, Pedone PV, Grimaldi G, Riccio A, et al. In embryonic stem cells, ZFP57/KAP1 recognize a methylated hexanucleotide to affect chromatin and DNA methylation of imprinting control regions. Mol Cell 2011; 44:361 - 72; http://dx.doi.org/10.1016/j.molcel.2011.08.032; PMID: 22055183
- García-García MJ, Shibata M, Anderson KV. Chato, a KRAB zinc-finger protein, regulates convergent extension in the mouse embryo. Development 2008; 135:3053 - 62; http://dx.doi.org/10.1242/dev.022897; PMID: 18701545
- Shibata M, García-García MJ. The mouse KRAB zinc-finger protein CHATO is required in embryonic-derived tissues to control yolk sac and placenta morphogenesis. Dev Biol 2011; 349:331 - 41; http://dx.doi.org/10.1016/j.ydbio.2010.11.015; PMID: 21094155
- Wolf D, Goff SP. Embryonic stem cells use ZFP809 to silence retroviral DNAs. Nature 2009; 458:1201 - 4; http://dx.doi.org/10.1038/nature07844; PMID: 19270682
- Li X, Ito M, Zhou F, Youngson N, Zuo X, Leder P, Ferguson-Smith AC. A maternal-zygotic effect gene, Zfp57, maintains both maternal and paternal imprints. Dev Cell 2008; 15:547 - 57; http://dx.doi.org/10.1016/j.devcel.2008.08.014; PMID: 18854139
- Tan X, Xu X, Elkenani M, Smorag L, Zechner U, Nolte J, Engel W, Pantakani DV. Zfp819, a novel KRAB-zinc finger protein, interacts with KAP1 and functions in genomic integrity maintenance of mouse embryonic stem cells. Stem Cell Res 2013; 11:1045 - 59; http://dx.doi.org/10.1016/j.scr.2013.07.006; PMID: 23954693
- Baudat F, Buard J, Grey C, Fledel-Alon A, Ober C, Przeworski M, Coop G, de Massy B. PRDM9 is a major determinant of meiotic recombination hotspots in humans and mice. Science 2010; 327:836 - 40; http://dx.doi.org/10.1126/science.1183439; PMID: 20044539
- Berg IL, Neumann R, Lam KW, Sarbajna S, Odenthal-Hesse L, May CA, Jeffreys AJ. PRDM9 variation strongly influences recombination hot-spot activity and meiotic instability in humans. Nat Genet 2010; 42:859 - 63; http://dx.doi.org/10.1038/ng.658; PMID: 20818382
- Flachs P, Mihola O, Simeček P, Gregorová S, Schimenti JC, Matsui Y, Baudat F, de Massy B, Piálek J, Forejt J, et al. Interallelic and intergenic incompatibilities of the Prdm9 (Hst1) gene in mouse hybrid sterility. PLoS Genet 2012; 8:e1003044; http://dx.doi.org/10.1371/journal.pgen.1003044; PMID: 23133405
- Mihola O, Trachtulec Z, Vlcek C, Schimenti JC, Forejt J. A mouse speciation gene encodes a meiotic histone H3 methyltransferase. Science 2009; 323:373 - 5; http://dx.doi.org/10.1126/science.1163601; PMID: 19074312
- Lupo A, Cesaro E, Montano G, Zurlo D, Izzo P, Costanzo P. KRAB-Zinc Finger Proteins: A Repressor Family Displaying Multiple Biological Functions. Curr Genomics 2013; 14:268 - 78; http://dx.doi.org/10.2174/13892029113149990002; PMID: 24294107
- Goodarzi AA, Noon AT, Deckbar D, Ziv Y, Shiloh Y, Löbrich M, Jeggo PA. ATM signaling facilitates repair of DNA double-strand breaks associated with heterochromatin. Mol Cell 2008; 31:167 - 77; http://dx.doi.org/10.1016/j.molcel.2008.05.017; PMID: 18657500
- Noon AT, Shibata A, Rief N, Löbrich M, Stewart GS, Jeggo PA, Goodarzi AA. 53BP1-dependent robust localized KAP-1 phosphorylation is essential for heterochromatic DNA double-strand break repair. Nat Cell Biol 2010; 12:177 - 84; http://dx.doi.org/10.1038/ncb2017; PMID: 20081839
- Ziv Y, Bielopolski D, Galanty Y, Lukas C, Taya Y, Schultz DC, Lukas J, Bekker-Jensen S, Bartek J, Shiloh Y. Chromatin relaxation in response to DNA double-strand breaks is modulated by a novel ATM- and KAP-1 dependent pathway. Nat Cell Biol 2006; 8:870 - 6; http://dx.doi.org/10.1038/ncb1446; PMID: 16862143
- Furuta S, Wang JM, Wei S, Jeng YM, Jiang X, Gu B, Chen PL, Lee EY, Lee WH. Removal of BRCA1/CtIP/ZBRK1 repressor complex on ANG1 promoter leads to accelerated mammary tumor growth contributed by prominent vasculature. Cancer Cell 2006; 10:13 - 24; http://dx.doi.org/10.1016/j.ccr.2006.05.022; PMID: 16843262
- Liao CC, Tsai CY, Chang WC, Lee WH, Wang JMRB. RB·E2F1 complex mediates DNA damage responses through transcriptional regulation of ZBRK1. J Biol Chem 2010; 285:33134 - 43; http://dx.doi.org/10.1074/jbc.M110.143461; PMID: 20713352
- Lin LF, Chuang CH, Li CF, Liao CC, Cheng CP, Cheng TL, Shen MR, Tseng JT, Chang WC, Lee WH, et al. ZBRK1 acts as a metastatic suppressor by directly regulating MMP9 in cervical cancer. Cancer Res 2010; 70:192 - 201; http://dx.doi.org/10.1158/0008-5472.CAN-09-2641; PMID: 19996286
- Zheng L, Pan H, Li S, Flesken-Nikitin A, Chen PL, Boyer TG, Lee WH. Sequence-specific transcriptional corepressor function for BRCA1 through a novel zinc finger protein, ZBRK1. Mol Cell 2000; 6:757 - 68; http://dx.doi.org/10.1016/S1097-2765(00)00075-7; PMID: 11090615
- Rubporn A, Srisomsap C, Subhasitanont P, Chokchaichamnankit D, Chiablaem K, Svasti J, Sangvanich P. Comparative proteomic analysis of lung cancer cell line and lung fibroblast cell line. Cancer Genomics Proteomics 2009; 6:229 - 37; PMID: 19657000
- Wang C, Ivanov A, Chen L, Fredericks WJ, Seto E, Rauscher FJ 3rd, Chen J. MDM2 interaction with nuclear corepressor KAP1 contributes to p53 inactivation. EMBO J 2005; 24:3279 - 90; http://dx.doi.org/10.1038/sj.emboj.7600791; PMID: 16107876
- Yang B, O’Herrin SM, Wu J, Reagan-Shaw S, Ma Y, Bhat KM, Gravekamp C, Setaluri V, Peters N, Hoffmann FM, et al. MAGE-A, mMage-b, and MAGE-C proteins form complexes with KAP1 and suppress p53-dependent apoptosis in MAGE-positive cell lines. Cancer Res 2007; 67:9954 - 62; http://dx.doi.org/10.1158/0008-5472.CAN-07-1478; PMID: 17942928
- Yokoe T, Toiyama Y, Okugawa Y, Tanaka K, Ohi M, Inoue Y, Mohri Y, Miki C, Kusunoki M. KAP1 is associated with peritoneal carcinomatosis in gastric cancer. Ann Surg Oncol 2010; 17:821 - 8; http://dx.doi.org/10.1245/s10434-009-0795-8; PMID: 19898899
- Tian C, Xing G, Xie P, Lu K, Nie J, Wang J, Li L, Gao M, Zhang L, He F. KRAB-type zinc-finger protein Apak specifically regulates p53-dependent apoptosis. Nat Cell Biol 2009; 11:580 - 91; http://dx.doi.org/10.1038/ncb1864; PMID: 19377469
- Yuan L, Tian C, Wang H, Song S, Li D, Xing G, Yin Y, He F, Zhang L. Apak competes with p53 for direct binding to intron 1 of p53AIP1 to regulate apoptosis. EMBO Rep 2012; 13:363 - 70; http://dx.doi.org/10.1038/embor.2012.10; PMID: 22334068
- Cecere G, Hoersch S, Jensen MB, Dixit S, Grishok A. The ZFP-1(AF10)/DOT-1 complex opposes H2B ubiquitination to reduce Pol II transcription. Mol Cell 2013; 50:894 - 907; http://dx.doi.org/10.1016/j.molcel.2013.06.002; PMID: 23806335
- Jakobsson J, Cordero MI, Bisaz R, Groner AC, Busskamp V, Bensadoun JC, Cammas F, Losson R, Mansuy IM, Sandi C, et al. KAP1-mediated epigenetic repression in the forebrain modulates behavioral vulnerability to stress. Neuron 2008; 60:818 - 31; http://dx.doi.org/10.1016/j.neuron.2008.09.036; PMID: 19081377
- Shin JH, Ko HS, Kang H, Lee Y, Lee YI, Pletinkova O, Troconso JC, Dawson VL, Dawson TM. PARIS (ZNF746) repression of PGC-1α contributes to neurodegeneration in Parkinson’s disease. Cell 2011; 144:689 - 702; http://dx.doi.org/10.1016/j.cell.2011.02.010; PMID: 21376232
- Bojkowska K, Aloisio F, Cassano M, Kapopoulou A, Santoni de Sio F, Zangger N, Offner S, Cartoni C, Thomas C, Quenneville S, et al. Liver-specific ablation of Krüppel-associated box-associated protein 1 in mice leads to male-predominant hepatosteatosis and development of liver adenoma. Hepatology 2012; 56:1279 - 90; http://dx.doi.org/10.1002/hep.25767; PMID: 22684873
- Krebs CJ, Larkins LK, Price R, Tullis KM, Miller RD, Robins DM. Regulator of sex-limitation (Rsl) encodes a pair of KRAB zinc-finger genes that control sexually dimorphic liver gene expression. Genes Dev 2003; 17:2664 - 74; http://dx.doi.org/10.1101/gad.1135703; PMID: 14563677
- Oliver CH, Khaled WT, Frend H, Nichols J, Watson CJ. The Stat6-regulated KRAB domain zinc finger protein Zfp157 regulates the balance of lineages in mammary glands and compensates for loss of Gata-3. Genes Dev 2012; 26:1086 - 97; http://dx.doi.org/10.1101/gad.184051.111; PMID: 22588720
- Barde I, Rauwel B, Marin-Florez RM, Corsinotti A, Laurenti E, Verp S, Offner S, Marquis J, Kapopoulou A, Vanicek J, et al. A KRAB/KAP1-miRNA cascade regulates erythropoiesis through stage-specific control of mitophagy. Science 2013; 340:350 - 3; http://dx.doi.org/10.1126/science.1232398; PMID: 23493425
- Rickert RC, Roes J, Rajewsky K. B lymphocyte-specific, Cre-mediated mutagenesis in mice. Nucleic Acids Res 1997; 25:1317 - 8; http://dx.doi.org/10.1093/nar/25.6.1317; PMID: 9092650
- Jeevan-Raj BP, Robert I, Heyer V, Page A, Wang JH, Cammas F, Alt FW, Losson R, Reina-San-Martin B. Epigenetic tethering of AID to the donor switch region during immunoglobulin class switch recombination. J Exp Med 2011; 208:1649 - 60; http://dx.doi.org/10.1084/jem.20110118; PMID: 21746811
- Revy P, Muto T, Levy Y, Geissmann F, Plebani A, Sanal O, Catalan N, Forveille M, Dufourcq-Labelouse R, Gennery A, et al. Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the Hyper-IgM syndrome (HIGM2). Cell 2000; 102:565 - 75; http://dx.doi.org/10.1016/S0092-8674(00)00079-9; PMID: 11007475
- Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell 2000; 102:553 - 63; http://dx.doi.org/10.1016/S0092-8674(00)00078-7; PMID: 11007474
- Xu Z, Zan H, Pone EJ, Mai T, Casali P. Immunoglobulin class-switch DNA recombination: induction, targeting and beyond. Nat Rev Immunol 2012; 12:517 - 31; http://dx.doi.org/10.1038/nri3216; PMID: 22728528
- Di Noia JM, Neuberger MS. Molecular mechanisms of antibody somatic hypermutation. Annu Rev Biochem 2007; 76:1 - 22; http://dx.doi.org/10.1146/annurev.biochem.76.061705.090740; PMID: 17328676
- Daniel JA, Nussenzweig A. The AID-induced DNA damage response in chromatin. Mol Cell 2013; 50:309 - 21; http://dx.doi.org/10.1016/j.molcel.2013.04.017; PMID: 23664375
- Santoni de Sio FR, Massacand J, Barde I, Offner S, Corsinotti A, Kapopoulou A, Bojkowska K, Dagklis A, Fernandez M, Ghia P, et al. KAP1 regulates gene networks controlling mouse B-lymphoid cell differentiation and function. Blood 2012; 119:4675 - 85; http://dx.doi.org/10.1182/blood-2011-12-401117; PMID: 22452978
- Baracho GV, Miletic AV, Omori SA, Cato MH, Rickert RC. Emergence of the PI3-kinase pathway as a central modulator of normal and aberrant B cell differentiation. Curr Opin Immunol 2011; 23:178 - 83; http://dx.doi.org/10.1016/j.coi.2011.01.001; PMID: 21277760
- Heintzman ND, Stuart RK, Hon G, Fu Y, Ching CW, Hawkins RD, Barrera LO, Van Calcar S, Qu C, Ching KA, et al. Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat Genet 2007; 39:311 - 8; http://dx.doi.org/10.1038/ng1966; PMID: 17277777
- Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, Cheng JX, Murre C, Singh H, Glass CK. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell 2010; 38:576 - 89; http://dx.doi.org/10.1016/j.molcel.2010.05.004; PMID: 20513432
- Chikuma S, Suita N, Okazaki IM, Shibayama S, Honjo T. TRIM28 prevents autoinflammatory T cell development in vivo. Nat Immunol 2012; 13:596 - 603; http://dx.doi.org/10.1038/ni.2293; PMID: 22544392
- Santoni de Sio FR, Barde I, Offner S, Kapopoulou A, Corsinotti A, Bojkowska K, Genolet R, Thomas JH, Luescher IF, Pinschewer D, et al. KAP1 regulates gene networks controlling T-cell development and responsiveness. FASEB J 2012; 26:4561 - 75; http://dx.doi.org/10.1096/fj.12-206177; PMID: 22872677
- Zhou XF, Yu J, Chang M, Zhang M, Zhou D, Cammas F, Sun SC. TRIM28 mediates chromatin modifications at the TCRα enhancer and regulates the development of T and natural killer T cells. Proc Natl Acad Sci U S A 2012; 109:20083 - 8; http://dx.doi.org/10.1073/pnas.1214704109; PMID: 23169648
- Kim J, Sif S, Jones B, Jackson A, Koipally J, Heller E, Winandy S, Viel A, Sawyer A, Ikeda T, et al. Ikaros DNA-binding proteins direct formation of chromatin remodeling complexes in lymphocytes. Immunity 1999; 10:345 - 55; http://dx.doi.org/10.1016/S1074-7613(00)80034-5; PMID: 10204490
- Lee SJ, Lee JR, Hahn HS, Kim YH, Ahn JH, Bae CD, Yang JM, Hahn MJ. PIAS1 interacts with the KRAB zinc finger protein, ZNF133, via zinc finger motifs and regulates its transcriptional activity. Exp Mol Med 2007; 39:450 - 7; http://dx.doi.org/10.1038/emm.2007.49; PMID: 17934332
- Yun J, Lee WH. Degradation of transcription repressor ZBRK1 through the ubiquitin-proteasome pathway relieves repression of Gadd45a upon DNA damage. Mol Cell Biol 2003; 23:7305 - 14; http://dx.doi.org/10.1128/MCB.23.20.7305-7314.2003; PMID: 14517299
- Harada Y, Kanehira M, Fujisawa Y, Takata R, Shuin T, Miki T, Fujioka T, Nakamura Y, Katagiri T. Cell-permeable peptide DEPDC1-ZNF224 interferes with transcriptional repression and oncogenicity in bladder cancer cells. Cancer Res 2010; 70:5829 - 39; http://dx.doi.org/10.1158/0008-5472.CAN-10-0255; PMID: 20587513
- Montano G, Cesaro E, Fattore L, Vidovic K, Palladino C, Crescitelli R, Izzo P, Turco MC, Costanzo P. Role of WT1-ZNF224 interaction in the expression of apoptosis-regulating genes. Hum Mol Genet 2013; 22:1771 - 82; http://dx.doi.org/10.1093/hmg/ddt027; PMID: 23362234
- Groner AC, Meylan S, Ciuffi A, Zangger N, Ambrosini G, Dénervaud N, Bucher P, Trono D. KRAB-zinc finger proteins and KAP1 can mediate long-range transcriptional repression through heterochromatin spreading. PLoS Genet 2010; 6:e1000869; http://dx.doi.org/10.1371/journal.pgen.1000869; PMID: 20221260
- Iyengar S, Ivanov AV, Jin VX, Rauscher FJ 3rd, Farnham PJ. Functional analysis of KAP1 genomic recruitment. Mol Cell Biol 2011; 31:1833 - 47; http://dx.doi.org/10.1128/MCB.01331-10; PMID: 21343339
- O’Geen H, Squazzo SL, Iyengar S, Blahnik K, Rinn JL, Chang HY, Green R, Farnham PJ. Genome-wide analysis of KAP1 binding suggests autoregulation of KRAB-ZNFs. PLoS Genet 2007; 3:e89; http://dx.doi.org/10.1371/journal.pgen.0030089; PMID: 17542650
- Vogel MJ, Guelen L, de Wit E, Peric-Hupkes D, Lodén M, Talhout W, Feenstra M, Abbas B, Classen AK, van Steensel B. Human heterochromatin proteins form large domains containing KRAB-ZNF genes. Genome Res 2006; 16:1493 - 504; http://dx.doi.org/10.1101/gr.5391806; PMID: 17038565
- Blahnik KR, Dou L, Echipare L, Iyengar S, O’Geen H, Sanchez E, Zhao Y, Marra MA, Hirst M, Costello JF, et al. Characterization of the contradictory chromatin signatures at the 3′ exons of zinc finger genes. PLoS One 2011; 6:e17121; http://dx.doi.org/10.1371/journal.pone.0017121; PMID: 21347206
- Thomas LR, Miyashita H, Cobb RM, Pierce S, Tachibana M, Hobeika E, Reth M, Shinkai Y, Oltz EM. Functional analysis of histone methyltransferase g9a in B and T lymphocytes. J Immunol 2008; 181:485 - 93; http://dx.doi.org/10.4049/jimmunol.181.1.485; PMID: 18566414
- Lehnertz B, Northrop JP, Antignano F, Burrows K, Hadidi S, Mullaly SC, Rossi FM, Zaph C. Activating and inhibitory functions for the histone lysine methyltransferase G9a in T helper cell differentiation and function. J Exp Med 2010; 207:915 - 22; http://dx.doi.org/10.1084/jem.20100363; PMID: 20421388
- Allan RS, Zueva E, Cammas F, Schreiber HA, Masson V, Belz GT, Roche D, Maison C, Quivy JP, Almouzni G, et al. An epigenetic silencing pathway controlling T helper 2 cell lineage commitment. Nature 2012; 487:249 - 53; http://dx.doi.org/10.1038/nature11173; PMID: 22763435
- Bracken AP, Dietrich N, Pasini D, Hansen KH, Helin K. Genome-wide mapping of Polycomb target genes unravels their roles in cell fate transitions. Genes Dev 2006; 20:1123 - 36; http://dx.doi.org/10.1101/gad.381706; PMID: 16618801
- De Santa F, Narang V, Yap ZH, Tusi BK, Burgold T, Austenaa L, Bucci G, Caganova M, Notarbartolo S, Casola S, et al. Jmjd3 contributes to the control of gene expression in LPS-activated macrophages. EMBO J 2009; 28:3341 - 52; http://dx.doi.org/10.1038/emboj.2009.271; PMID: 19779457
- Scacheri PC, Davis S, Odom DT, Crawford GE, Perkins S, Halawi MJ, Agarwal SK, Marx SJ, Spiegel AM, Meltzer PS, et al. Genome-wide analysis of menin binding provides insights into MEN1 tumorigenesis. PLoS Genet 2006; 2:e51; http://dx.doi.org/10.1371/journal.pgen.0020051; PMID: 16604156
- Sridharan R, Smale ST. Predominant interaction of both Ikaros and Helios with the NuRD complex in immature thymocytes. J Biol Chem 2007; 282:30227 - 38; http://dx.doi.org/10.1074/jbc.M702541200; PMID: 17681952
- Zhang J, Jackson AF, Naito T, Dose M, Seavitt J, Liu F, Heller EJ, Kashiwagi M, Yoshida T, Gounari F, et al. Harnessing of the nucleosome-remodeling-deacetylase complex controls lymphocyte development and prevents leukemogenesis. Nat Immunol 2012; 13:86 - 94; http://dx.doi.org/10.1038/ni.2150; PMID: 22080921
- Bandyopadhyay S, Montagna C, Macian F. Silencing of the Il2 gene transcription is regulated by epigenetic changes in anergic T cells. Eur J Immunol 2012; 42:2471 - 83; http://dx.doi.org/10.1002/eji.201142307; PMID: 22684523
- Fujita N, Jaye DL, Geigerman C, Akyildiz A, Mooney MR, Boss JM, Wade PA. MTA3 and the Mi-2/NuRD complex regulate cell fate during B lymphocyte differentiation. Cell 2004; 119:75 - 86; http://dx.doi.org/10.1016/j.cell.2004.09.014; PMID: 15454082
- Gao H, Lukin K, Ramírez J, Fields S, Lopez D, Hagman J. Opposing effects of SWI/SNF and Mi-2/NuRD chromatin remodeling complexes on epigenetic reprogramming by EBF and Pax5. Proc Natl Acad Sci U S A 2009; 106:11258 - 63; http://dx.doi.org/10.1073/pnas.0809485106; PMID: 19549820
- Harker N, Naito T, Cortes M, Hostert A, Hirschberg S, Tolaini M, Roderick K, Georgopoulos K, Kioussis D. The CD8alpha gene locus is regulated by the Ikaros family of proteins. Mol Cell 2002; 10:1403 - 15; http://dx.doi.org/10.1016/S1097-2765(02)00711-6; PMID: 12504015
- Lu X, Kovalev GI, Chang H, Kallin E, Knudsen G, Xia L, Mishra N, Ruiz P, Li E, Su L, et al. Inactivation of NuRD component Mta2 causes abnormal T cell activation and lupus-like autoimmune disease in mice. J Biol Chem 2008; 283:13825 - 33; http://dx.doi.org/10.1074/jbc.M801275200; PMID: 18353770
- Naito T, Gómez-Del Arco P, Williams CJ, Georgopoulos K. Antagonistic interactions between Ikaros and the chromatin remodeler Mi-2beta determine silencer activity and Cd4 gene expression. Immunity 2007; 27:723 - 34; http://dx.doi.org/10.1016/j.immuni.2007.09.008; PMID: 17980631
- Williams CJ, Naito T, Arco PG, Seavitt JR, Cashman SM, De Souza B, Qi X, Keables P, Von Andrian UH, Georgopoulos K. The chromatin remodeler Mi-2beta is required for CD4 expression and T cell development. Immunity 2004; 20:719 - 33; http://dx.doi.org/10.1016/j.immuni.2004.05.005; PMID: 15189737
- Watanabe A, Higuchi M, Fukushi M, Ohsawa T, Takahashi M, Oie M, Fujii M. A novel KRAB-Zinc finger protein interacts with latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus and activates transcription via terminal repeat sequences. Virus Genes 2007; 34:127 - 36; http://dx.doi.org/10.1007/s11262-006-0048-x; PMID: 17143723
- Chang PC, Fitzgerald LD, Van Geelen A, Izumiya Y, Ellison TJ, Wang DH, Ann DK, Luciw PA, Kung HJ. Kruppel-associated box domain-associated protein-1 as a latency regulator for Kaposi’s sarcoma-associated herpesvirus and its modulation by the viral protein kinase. Cancer Res 2009; 69:5681 - 9; http://dx.doi.org/10.1158/0008-5472.CAN-08-4570; PMID: 19584288
- Yang Z, Wen H-J, Minhas V, Wood C. The zinc finger DNA-binding domain of K-RBP plays an important role in regulating Kaposi’s sarcoma-associated herpesvirus RTA-mediated gene expression. Virology 2009; 391:221 - 31; http://dx.doi.org/10.1016/j.virol.2009.06.014; PMID: 19592062
- Liao G, Huang J, Fixman ED, Hayward SD. The Epstein-Barr virus replication protein BBLF2/3 provides an origin-tethering function through interaction with the zinc finger DNA binding protein ZBRK1 and the KAP-1 corepressor. J Virol 2005; 79:245 - 56; http://dx.doi.org/10.1128/JVI.79.1.245-256.2005; PMID: 15596820
- Carlson KA, Leisman G, Limoges J, Pohlman GD, Horiba M, Buescher J, Gendelman HE, Ikezu T. Molecular characterization of a putative antiretroviral transcriptional factor, OTK18. J Immunol 2004; 172:381 - 91; http://dx.doi.org/10.4049/jimmunol.172.1.381; PMID: 14688346
- Horiba M, Martinez LB, Buescher JL, Sato S, Limoges J, Jiang Y, Jones C, Ikezu T. OTK18, a zinc-finger protein, regulates human immunodeficiency virus type 1 long terminal repeat through two distinct regulatory regions. J Gen Virol 2007; 88:236 - 41; http://dx.doi.org/10.1099/vir.0.82066-0; PMID: 17170456
- Nishitsuji H, Abe M, Sawada R, Takaku H. ZBRK1 represses HIV-1 LTR-mediated transcription. FEBS Lett 2012; 586:3562 - 8; http://dx.doi.org/10.1016/j.febslet.2012.08.010; PMID: 22975076
- Allouch A, Di Primio C, Alpi E, Lusic M, Arosio D, Giacca M, Cereseto A. The TRIM family protein KAP1 inhibits HIV-1 integration. Cell Host Microbe 2011; 9:484 - 95; http://dx.doi.org/10.1016/j.chom.2011.05.004; PMID: 21669397
- Bednarski JJ, Sleckman BP. Lymphocyte development: integration of DNA damage response signaling. Adv Immunol 2012; 116:175 - 204; http://dx.doi.org/10.1016/B978-0-12-394300-2.00006-5; PMID: 23063077
- Gapud EJ, Dorsett Y, Yin B, Callen E, Bredemeyer A, Mahowald GK, Omi KQ, Walker LM, Bednarski JJ, McKinnon PJ, et al. Ataxia telangiectasia mutated (Atm) and DNA-PKcs kinases have overlapping activities during chromosomal signal joint formation. Proc Natl Acad Sci U S A 2011; 108:2022 - 7; http://dx.doi.org/10.1073/pnas.1013295108; PMID: 21245316
- Zha S, Jiang W, Fujiwara Y, Patel H, Goff PH, Brush JW, Dubois RL, Alt FW. Ataxia telangiectasia-mutated protein and DNA-dependent protein kinase have complementary V(D)J recombination functions. Proc Natl Acad Sci U S A 2011; 108:2028 - 33; http://dx.doi.org/10.1073/pnas.1019293108; PMID: 21245310
- Woodbine L, Neal JA, Sasi NK, Shimada M, Deem K, Coleman H, Dobyns WB, Ogi T, Meek K, Davies EG, et al. PRKDC mutations in a SCID patient with profound neurological abnormalities. J Clin Invest 2013; 123:2969 - 80; http://dx.doi.org/10.1172/JCI67349; PMID: 23722905
- van der Burg M, Ijspeert H, Verkaik NS, Turul T, Wiegant WW, Morotomi-Yano K, Mari PO, Tezcan I, Chen DJ, Zdzienicka MZ, et al. A DNA-PKcs mutation in a radiosensitive T-B- SCID patient inhibits Artemis activation and nonhomologous end-joining. J Clin Invest 2009; 119:91 - 8; PMID: 19075392
- Lavin MF, Shiloh Y. The genetic defect in ataxia-telangiectasia. Annu Rev Immunol 1997; 15:177 - 202; http://dx.doi.org/10.1146/annurev.immunol.15.1.177; PMID: 9143686
- Nowak-Wegrzyn A, Crawford TO, Winkelstein JA, Carson KA, Lederman HM. Immunodeficiency and infections in ataxia-telangiectasia. J Pediatr 2004; 144:505 - 11; http://dx.doi.org/10.1016/j.jpeds.2003.12.046; PMID: 15069401
- Liyanage M, Weaver Z, Barlow C, Coleman A, Pankratz DG, Anderson S, Wynshaw-Boris A, Ried T. Abnormal rearrangement within the alpha/delta T-cell receptor locus in lymphomas from Atm-deficient mice. Blood 2000; 96:1940 - 6; PMID: 10961898
- Xu Y, Ashley T, Brainerd EE, Bronson RT, Meyn MS, Baltimore D. Targeted disruption of ATM leads to growth retardation, chromosomal fragmentation during meiosis, immune defects, and thymic lymphoma. Genes Dev 1996; 10:2411 - 22; http://dx.doi.org/10.1101/gad.10.19.2411; PMID: 8843194
- Isoda T, Takagi M, Piao J, Nakagama S, Sato M, Masuda K, Ikawa T, Azuma M, Morio T, Kawamoto H, et al. Process for immune defect and chromosomal translocation during early thymocyte development lacking ATM. Blood 2012; 120:789 - 99; http://dx.doi.org/10.1182/blood-2012-02-413195; PMID: 22709691
- Matei IR, Gladdy RA, Nutter LM, Canty A, Guidos CJ, Danska JS. ATM deficiency disrupts Tcra locus integrity and the maturation of CD4+CD8+ thymocytes. Blood 2007; 109:1887 - 96; http://dx.doi.org/10.1182/blood-2006-05-020917; PMID: 17077325
- Vacchio MS, Olaru A, Livak F, Hodes RJ. ATM deficiency impairs thymocyte maturation because of defective resolution of T cell receptor alpha locus coding end breaks. Proc Natl Acad Sci U S A 2007; 104:6323 - 8; http://dx.doi.org/10.1073/pnas.0611222104; PMID: 17405860
- Hewitt SL, Yin B, Ji Y, Chaumeil J, Marszalek K, Tenthorey J, Salvagiotto G, Steinel N, Ramsey LB, Ghysdael J, et al. RAG-1 and ATM coordinate monoallelic recombination and nuclear positioning of immunoglobulin loci. Nat Immunol 2009; 10:655 - 64; http://dx.doi.org/10.1038/ni.1735; PMID: 19448632
- Ruan Q, Chen YH. Nuclear factor-κB in immunity and inflammation: the Treg and Th17 connection. Adv Exp Med Biol 2012; 946:207 - 21; http://dx.doi.org/10.1007/978-1-4614-0106-3_12; PMID: 21948370
- Chen L-F, Greene WC. Shaping the nuclear action of NF-kappaB. Nat Rev Mol Cell Biol 2004; 5:392 - 401; http://dx.doi.org/10.1038/nrm1368; PMID: 15122352
- Kamitani S, Togi S, Ikeda O, Nakasuji M, Sakauchi A, Sekine Y, Muromoto R, Oritani K, Matsuda T. Krüppel-associated box-associated protein 1 negatively regulates TNF-α-induced NF-κB transcriptional activity by influencing the interactions among STAT3, p300, and NF-κB/p65. J Immunol 2011; 187:2476 - 83; http://dx.doi.org/10.4049/jimmunol.1003243; PMID: 21810609
- Tsuruma R, Ohbayashi N, Kamitani S, Ikeda O, Sato N, Muromoto R, Sekine Y, Oritani K, Matsuda T. Physical and functional interactions between STAT3 and KAP1. Oncogene 2008; 27:3054 - 9; http://dx.doi.org/10.1038/sj.onc.1210952; PMID: 18037959
- Kamitani S, Ohbayashi N, Ikeda O, Togi S, Muromoto R, Sekine Y, Ohta K, Ishiyama H, Matsuda T. KAP1 regulates type I interferon/STAT1-mediated IRF-1 gene expression. Biochem Biophys Res Commun 2008; 370:366 - 70; http://dx.doi.org/10.1016/j.bbrc.2008.03.104; PMID: 18381204
- Chakravarti D, Ogryzko V, Kao HY, Nash A, Chen H, Nakatani Y, Evans RM. A viral mechanism for inhibition of p300 and PCAF acetyltransferase activity. Cell 1999; 96:393 - 403; http://dx.doi.org/10.1016/S0092-8674(00)80552-8; PMID: 10025405
- Korzus E, Torchia J, Rose DW, Xu L, Kurokawa R, McInerney EM, Mullen TM, Glass CK, Rosenfeld MG. Transcription factor-specific requirements for coactivators and their acetyltransferase functions. Science 1998; 279:703 - 7; http://dx.doi.org/10.1126/science.279.5351.703; PMID: 9445475
- O’Shea JJ, Plenge R. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity 2012; 36:542 - 50; http://dx.doi.org/10.1016/j.immuni.2012.03.014; PMID: 22520847
- Chen Z, Laurence A, Kanno Y, Pacher-Zavisin M, Zhu BM, Tato C, Yoshimura A, Hennighausen L, O’Shea JJ. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc Natl Acad Sci U S A 2006; 103:8137 - 42; http://dx.doi.org/10.1073/pnas.0600666103; PMID: 16698929
- Durant L, Watford WT, Ramos HL, Laurence A, Vahedi G, Wei L, Takahashi H, Sun HW, Kanno Y, Powrie F, et al. Diverse targets of the transcription factor STAT3 contribute to T cell pathogenicity and homeostasis. Immunity 2010; 32:605 - 15; http://dx.doi.org/10.1016/j.immuni.2010.05.003; PMID: 20493732
- Ma CS, Chew GY, Simpson N, Priyadarshi A, Wong M, Grimbacher B, Fulcher DA, Tangye SG, Cook MC. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J Exp Med 2008; 205:1551 - 7; http://dx.doi.org/10.1084/jem.20080218; PMID: 18591410
- Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y, Kas A, Rudensky AY. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science 2009; 326:986 - 91; http://dx.doi.org/10.1126/science.1172702; PMID: 19797626
- Laurence A, Amarnath S, Mariotti J, Kim YC, Foley J, Eckhaus M, O’Shea JJ, Fowler DH. STAT3 transcription factor promotes instability of nTreg cells and limits generation of iTreg cells during acute murine graft-versus-host disease. Immunity 2012; 37:209 - 22; http://dx.doi.org/10.1016/j.immuni.2012.05.027; PMID: 22921119
- Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet 2001; 27:18 - 20; http://dx.doi.org/10.1038/83707; PMID: 11137992
- Passerini L, Santoni de Sio FR, Roncarolo MG, Bacchetta R. Forkhead box P3: The Peacekeeper of the Immune System. Int Rev Immunol 2013; PMID: 24354325
- Katoh H, Zheng P, Liu Y. FOXP3: genetic and epigenetic implications for autoimmunity. J Autoimmun 2013; 41:72 - 8; http://dx.doi.org/10.1016/j.jaut.2012.12.004; PMID: 23313429
- Hossain DM, Panda AK, Manna A, Mohanty S, Bhattacharjee P, Bhattacharyya S, Saha T, Chakraborty S, Kar RK, Das T, et al. FoxP3 acts as a cotranscription factor with STAT3 in tumor-induced regulatory T cells. Immunity 2013; 39:1057 - 69; http://dx.doi.org/10.1016/j.immuni.2013.11.005; PMID: 24315995
- Huang C, Martin S, Pfleger C, Du J, Buckner JH, Bluestone JA, Riley JL, Ziegler SF. Cutting Edge: a novel, human-specific interacting protein couples FOXP3 to a chromatin-remodeling complex that contains KAP1/TRIM28. J Immunol 2013; 190:4470 - 3; http://dx.doi.org/10.4049/jimmunol.1203561; PMID: 23543754
- Chen X, El Gazzar M, Yoza BK, McCall CE. The NF-kappaB factor RelB and histone H3 lysine methyltransferase G9a directly interact to generate epigenetic silencing in endotoxin tolerance. J Biol Chem 2009; 284:27857 - 65; http://dx.doi.org/10.1074/jbc.M109.000950; PMID: 19690169
- Miao F, Smith DD, Zhang L, Min A, Feng W, Natarajan R. Lymphocytes from patients with type 1 diabetes display a distinct profile of chromatin histone H3 lysine 9 dimethylation: an epigenetic study in diabetes. Diabetes 2008; 57:3189 - 98; http://dx.doi.org/10.2337/db08-0645; PMID: 18776137
- Sharma P, Azebi S, England P, Christensen T, Møller-Larsen A, Petersen T, Batsché E, Muchardt C. Citrullination of histone H3 interferes with HP1-mediated transcriptional repression. PLoS Genet 2012; 8:e1002934; http://dx.doi.org/10.1371/journal.pgen.1002934; PMID: 23028349
- Zhao M, Wu X, Zhang Q, Luo S, Liang G, Su Y, Tan Y, Lu Q. RFX1 regulates CD70 and CD11a expression in lupus T cells by recruiting the histone methyltransferase SUV39H1. Arthritis Res Ther 2010; 12:R227; http://dx.doi.org/10.1186/ar3214; PMID: 21192791
- Cuddapah S, Barski A, Zhao K. Epigenomics of T cell activation, differentiation, and memory. Curr Opin Immunol 2010; 22:341 - 7; http://dx.doi.org/10.1016/j.coi.2010.02.007; PMID: 20226645
- Li G, Zan H, Xu Z, Casali P. Epigenetics of the antibody response. Trends Immunol 2013; 34:460 - 70; http://dx.doi.org/10.1016/j.it.2013.03.006; PMID: 23643790
