JNK signaling is needed to tolerate chromosomal instability
Chromosomal instability (CIN) is a common feature of tumors, thus representing a potential therapeutic target, if ways could be found to specifically cause apoptosis in genomically unstable dividing cells. It has previously been shown that downregulation of signaling through the Jun N-terminal kinase (JNK) pathway triggers apoptosis in models of CIN induced by loss of the spindle checkpoint. In a recent follow-up study, led by Drs Robert Saint and Stephen Gregory, the components upstream and downstream of JNK were identified that are able to mediate this effect. The authors also tested the involvement of p53 and DNA damage in apoptosis upon JNK signaling reduction in CIN cells. They found that cell cycle progression timing has a strong effect on apoptosis induced by downregulation of JNK signaling in genetically unstable cells: a shortened G2 Phase enhanced apoptosis, while lengthening G2 rescued the JNK-deficient CIN cell death phenotype. The findings suggest that chromosomal instability represents a significant stress to dividing cells and that, without JNK signaling, cells undergo apoptosis because they lack a timely and effective response to DNA damage ().
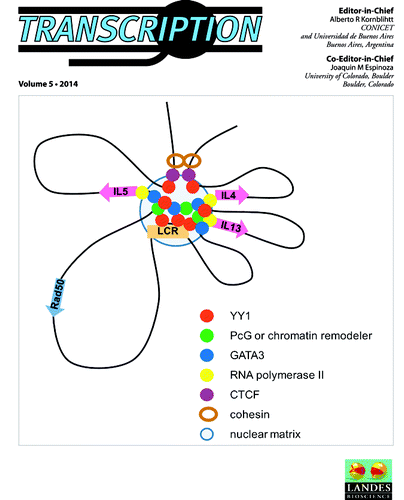
Physical mechanisms behind the large scale features of chromatin organization
Chromosomes in the nucleus of eukaryotic cells are arranged in a complex three-dimensional architecture. A recent review by Drs Ana Pombo and Mario Nicodemi summarizes and discusses recently published models of classical polymer physics of the general features of chromatin large scale spatial organization. Microscopic approaches, for instance, have shown that chromosomes occupy discrete nuclear regions called chromosomal territories. More recently, 3C-based technologies, such as Hi-C, have allowed us to derive detailed genome-wide contact matrices of the frequency of physical contacts between all genomic regions. The emerging intricate network has fascinating properties. One striking feature is the discovery that each chromosome is subdivided in Mb-sized domains (named TADs), characterized by enriched levels of interactions. Understanding the structure of the nucleus, the origin of the observed chromatin organization patterns, the factors that shape them, and how they are regulated by the cell for functional purposes are key challenges for scientists studying the eukaryotic nucleus. A combination of experimental and modeling approaches can convey a deeper understanding of the principles of genome function and, in the long run, of related diseases ().
Nuclear-specific roles for mTORC1 signaling
Mechanistic target of rapamycin complex 1 (mTORC1) is a well-known regulator of cell growth and proliferation in response to environmental stimuli and stressors. To date, the majority of mTORC1 studies have focused on its function as a cytoplasmic effector of translation regulation. More recently, several studies have identified additional, nuclear-specific roles for mTORC1 signaling related to transcription of the rDNA (rDNA) and ribosomal protein (RP) genes, mitotic cell cycle control, and the regulation of epigenetic processes. In a recent review, Dr Nicholas Laribee and colleagues highlight these significant findings and discuss the relevance of nuclear mTORC1 signaling dysregulation as it pertains to health and disease. The review also provides a summary of the main components of the mTOR pathway, focusing specifically on the mTORC1 branch. Since the majority of the mTORC1 signaling pathway has been defined in budding yeast and mammalian cells, the authors mainly cite studies using these two model systems, adding a few examples from other model systems where appropriate ().
DNA methylation and polycomb-mediated repression contribute to RARB silencing
Epigenetic repression is a common cellular mechanism to silence gene expression in mammalian cells. Two major actors in this process are DNA methylation and polycomb proteins. Both repressing systems are well described, but there are still many open questions about their interplay. The two repressor systems have often been described as mutually exclusive, but this statement is today controversial, as recent in vitro studies suggest the co-existence of both systems. A new study by Dr Paola Arimondo and colleagues has addressed this issue by looking at the Retinoic Acid Receptor β (RARβ) gene, a tumor suppressor gene frequently silenced in prostate cancer. They found that the RARβ promoter is hypermethylated in all studied prostate tumors and methylation levels are positively correlated with enrichments of polycomb-mediated H3K27me3. Thus, by using bisulfite conversion and pyrosequencing of immunoprecipitated H3K27me3 chromatin, the authors demonstrated that DNA methylation and polycomb repression co-exist in vivo at this locus. This repressive association was found in 6 of 6 patient tumor samples of different stages, suggesting a strong interplay of DNA methylation and the histone methyltransferase EZH2 (a member of the polycomb-group) to silence RARβ during prostate tumorigenesis. The data provide the first evidence that H3K27me3 and DNA methylation are related and co-exist in CpG rich regions in primary tissue samples from prostate cancer patients ().
References
- Wong HW-S, Shaukat Z, Wang J, Saint R, Gregory SL. JNK signaling is needed to tolerate chromosomal instability. Cell Cycle 2014; 13:622 - 31; http://dx.doi.org/10.4161/cc.27484; PMID: 24335260
References
- Pombo A, Nicodemi M. Physical mechanisms behind the large scale features of chromatin organization. Transcr 2014; 5
References
- Workman JJ, Chen H, Laribee RN. Environmental signaling through the mechanistic target of rapamycin complex 1: mTORC1 goes nuclear. Cell Cycle 2014; 13:714 - 25; http://dx.doi.org/10.4161/cc.28112; PMID: 24526113
References
- Moison C, Assemat F, Daunay A, Tost J, Guieysse-Peugeot AL, Arimondo PB. Synergistic chromatin repression of the tumor suppressor gene RARB in human prostate cancers. Epigenetics 2014; 9:477 - 82; http://dx.doi.org/10.4161/epi.27869; PMID: 24492483