Abstract
Transfer of a somatic nucleus into an enucleated oocyte is the most efficient approach for somatic cell reprogramming. While this process is known to involve extensive chromatin remodeling of the donor nucleus, the maternal factors responsible and the underlying chromatin-based mechanisms remain largely unknown. Here we discuss our recent findings demonstrating that the histone variant H3.3 plays an essential role in reprogramming and is required for reactivation of key pluripotency genes in somatic cell nuclear transfer (SCNT) embryos. Maternal-derived H3.3 replaces H3 in the donor nucleus shortly after oocyte activation, with the amount of replacement directly related to the differentiation status of the donor nucleus in SCNT embryos. We provide additional evidence to suggest that de novo synthesized H3.3 replaces histone H3 carrying repressive modifications in the donor nuclei of SCNT embryos, and hypothesize that replacement may occur at specific loci that must be reprogrammed for gene reactivation.
Introduction
The epigenome is established through patterns of DNA methylation, histone modification, and incorporation of histone variants, the regulation of which has been associated with gene expression changes that facilitate cell-type specification during development.Citation1 One of the most dramatic events in development occurs upon fertilization, resulting in epigenetic reprogramming of both the maternal and paternal genomes supported mainly by maternal components stored in the oocyte.Citation2 Intriguingly, the oocyte cytoplasm is also able to epigenetically reprogram differentiated somatic nuclei to a pluripotent state with the potential to differentiate into multiple lineages using somatic cell nuclear transfer (SCNT).Citation3-Citation6
Upon introduction of a somatic nucleus into an enucleated oocyte, the donor nucleus undergoes a series of reprogramming events initiated by maternal factors. The most obvious response of the donor nucleus to reprogramming is a large increase in volume, accompanied by the exchange of proteins between nucleus and cytoplasm. About 80% of the proteins brought in with the donor nucleus are lost within a few hours, and there is an obvious migration of maternal proteins into the donor nucleus.Citation7 During this process, many proteins from the somatic nucleus are exchanged for oocyte-specific factors. For example, the oocyte-specific linker histone H1foo replaces the somatic linker histone H1 in donor somatic nuclei,Citation8-Citation10 resulting in a change in composition of the chromatin. In addition, somatic components can be exchanged for the same component derived from the oocyte (e.g., HP1, BMI1, H3.3; note that maternal H3.3 can facilitate exchange of all H3 isoforms from the donor nucleus),Citation11-Citation13 resulting in a possible change in the modification state or conformation of the donor chromatin. Recent studies suggest that some protein replacement (e.g., H1, H3.3) is critical for pluripotency gene reactivation in the donor nucleus.Citation8,Citation13,Citation14 However, it remains unclear how identical protein isoforms are distinguished during replacement, and we speculate that one difference could be in the post-translational modification state of maternal-derived vs. somatic-derived proteins.
In addition to protein exchange, nuclear reprogramming involves additional restructuring of chromatin including changes in DNA and histone modification states and incorporation of histone variants into chromatin of the donor nuclei.Citation2 Remodeling of the donor chromatin landscape occurs with variable efficiency. Reprogramming of donor DNA methylation appears inefficient and incomplete, as cloned embryos contain DNA methylation patterns more similar to donor cells in various genomic regions than to normal embryos.Citation15 Additionally, imprinted genes are likely resistant to reprogramming by nuclear transfer.Citation16,Citation17 In contrast, the reprogramming of histone variants and histone modifications by nuclear transfer is believed to be much more efficient, with studies demonstrating that histone modifications in SCNT embryos recapitulate the patterns found in fertilized embryos in early embryonic stages.Citation18,Citation19
Following nuclear remodeling, there is a switch in gene expression profile in the donor nucleus from somatic to embryonic, requiring silencing of somatic expression and reactivation of embryonic genes, including pluripotency genes such as Pou5f1 (Oct4) and Nanog.Citation20 Though cloned embryos from mice can develop to the blastocyst stage with a high success rate (40–50%), the majority of these embryos are abnormal and die in the early embryonic stage after embryo transfer, with only 1–4% of the cloned embryos able to develop to term.Citation6,Citation21 Several studies have demonstrated that silencing of donor cell-type specific gene expression is incomplete in cloned embryos,Citation22,Citation23 perhaps providing an explanation for this low efficiency. Notably, one study suggests that incomplete silencing of the donor cell-type specific gene expression program might be related to failure to erase epigenetic memory marked by the histone variant H3.3 at the promoter regions of these genes.Citation23
The mechanism by which a silenced gene is activated in SCNT embryos remains a central question for nuclear reprogramming. In a proposed model,Citation24 the promoter of a silenced gene is marked by repression-associated modifications such as trimethylation of Lys9 (H3K9me3) and Lys27 (H3K27me3) on histone H3 which allow binding of heterochromatin protein-1 (HP1) and the polycomb repressive complex-1 (PRC1), respectively. Following nuclear transfer, embryonic transcription factors from the ooplasm bind to their target sites in the donor nucleus leading to the recruitment of chromatin-modifying activities and the establishment of a basal promoter complex. In support, the recruitment of Lys-specific histone demethylase-1 (LSD1), which demethylates H3K9, can occur in a transcription-factor-dependent manner. However, it is not known how H3K27 is demethylated or removed.Citation24 Additionally, this model does not consider chromatin remodeling and histone replacement (i.e., protein exchange described above) that can result in changes in nucleosome structure and histone modification at specific loci in SCNT embryos.
Our recent studies suggest that the histone variant H3.3 is an oocyte exchange factor that plays an important role in chromatin reorganization resulting in changes in gene expression during somatic nuclear reprogramming.Citation13 In mammals, the histone variant H3.3 is encoded by two different genes (h3f3a and h3f3b) whose translation results in an identical protein product (H3.3A and H3.3B) that differs from the canonical H3 isoforms by only 4 or 5 amino acids.Citation25-Citation27 Maternal H3.3 is incorporated into the decondensing sperm nucleus and is required for formation of the male pronucleus during fertilization,Citation28-Citation31 and is critical for the development and genomic integrity of pre-implantation embryos during early embryogenesis.Citation32 In addition, deposition of this replacement histone offers the potential to alter chromatin landscapes without the need for enzymatic removal of histone modifications. Here we discuss our recent study demonstrating the importance of maternal H3.3 during normal development and SCNT,Citation13 and offer additional mechanistic insight into the role of this histone variant and its effect on repressive histone modifications during the reprogramming process.
H3.3 is Required for Embryonic Development of Parthenogenetically Activated (PA) Embryos and Somatic Cell Nuclear Transfer (SCNT) Embryos
A variety of maternally transcribed mRNAs supplied by maternal cells are accumulated in mRNP (message ribonucleoprotein) complexes during oocyte maturation, and these mRNAs can be stored and localized within a specific region of the cytoplasm, or homogeneously dispersed within the cytoplasm of the entire oocyte.Citation33 Our data showed that the cytoplasm of mature mouse oocytes is abundant with both H3.3A and H3.3B mRNAs. These maternal mRNAs are gradually degraded upon oocyte activation and become largely depleted by the first embryonic cleavage (20 h after oocyte activation), with zygotic H3.3 elevated after the 2-cell stage. Direct microinjection of small interfering RNAs (siRNAs) against both H3.3A and H3.3B (H3.3 knockdown; referred to hereafter as H3.3KD) into metaphase II (MII) oocytes before activation can specifically knock down maternal H3.3 mRNAs. H3.3 depletion results in significantly compromised embryonic development of parthenogenetically activated (PA) embryos, with most of the H3.3KD embryos arresting at the late morula or early blastocyst stage. Maternal H3.3 is also a critical factor for the development of SCNT embryos, with embryos arresting at the 2- or 4-cell stage when maternal H3.3 is depleted before nuclear transfer. Addback of exogenous H3.3 mRNA, but not H3.2 mRNA, into H3.3KD oocytes restores reprogramming capacity, demonstrating the importance of the histone variant itself, rather than a loss of overall histone levels. Intriguingly, the maternally stored H3.3 mRNA in mature oocytes is sufficient to support early embryonic development to blastocyst stage and to derive embryonic stem cell (ESC) lines in the absence of zygotic H3.3 expression, demonstrated by the ability of a wild-type oocyte to reprogram a fibroblast nucleus devoid of H3.3. This result additionally indicates that the presence of H3.3 protein in the donor nucleus is not required for the nucleus to be reprogrammed.
Reactivation of Pluripotency-Associated Genes is Dependent upon Maternal H3.3 in SCNT Embryos
Pluripotency gene reactivation is a major event during somatic cell reprogramming.Citation20 Our study demonstrated that the presence of maternal H3.3 was required for activation of Oct4, a master regulator of pluripotency, from the donor nucleus. We injected a fibroblast nucleus containing GFP under control of the Oct4 promoter (Oct4-GFP) into one blastomere of a 2-cell PA embryo and found that the Oct4 gene of the fibroblast nucleus remains inactive in the resulting H3.3KD pre-implantation embryos. Interestingly, if an Oct4-EGFP ESC nucleus is injected, the Oct4 gene of the ESC nucleus is found normally expressed and not affected by the depletion of maternal H3.3 in oocytes. This result indicates that maternal H3.3 is specifically involved in the reactivation of gene expression in SCNT embryos during reprogramming and may not be required for the maintenance of gene expression, consistent with our observation of the maintenance of self-renewal in H3.3KD ESCs.Citation34 RNA-seq confirmed that many key pluripotency genes (e.g., Oct4, Nanog) fail to become activated upon SCNT in the absence of H3.3.
Maternal H3.3 Replaces Donor Nuclear H3 and is Associated with Global Reduction of H3K27me3 in the Nuclei of SCNT Embryos
To understand the mechanistic role of H3.3 in reactivation of pluripotency genes during somatic cell reprogramming, we isolated cumulus cells from a mouse model in which the endogenous H3.3B gene was edited to incorporate a C-terminal HA epitope tag (H3.3B-HA). H3.3B-HA cumulus cells were used for nuclear transfer and donor nuclear H3.3 protein in the nuclei of SCNT embryos was monitored using an HA antibody. We found that maternally derived H3.3 protein is gradually incorporated into the donor nucleus, while donor nucleus-derived H3.3 protein is lost in SCNT embryos beginning at 1–2 h of activation. The presence of H3.3 protein in the donor nucleus is not required for the incorporation of maternally derived H3.3 protein into the donor nucleus. Remarkably, the loss of donor H3.3 protein is related to the nuclear state of donor cells in that a higher proportion of the donor H3.3 protein is lost from differentiated donor nuclei than from pluripotent donor nuclei during reprogramming in SCNT embryos. This result shows that the loss of donor nucleus-derived H3.3 protein is not a global effect but is instead dependent upon the identity of the donor nucleus. Further, our data also indicates that the donor nucleus, rather than the ooplasm of the SCNT embryo, contains the signal for H3.3 replacement during reprogramming.
Collectively, our data suggests that de novo synthesized maternal H3.3 incorporation into donor nuclei is associated with gene reactivation and regulated by the identity, and perhaps the chromatin landscape, of the donor nuclei. It is well established that posttranslational modification (PTM) of histone tails is associated with transcriptional status, with trimethylation of histone 3 at lysine 4 (H3K4me3) associated with active genesCitation35 and H3K27me3 most often associated with gene repression,Citation36 and that many pluripotency genes acquire H3K27me3 at their regulatory regions over the course of differentiation. We hypothesized that to reactivate silenced genes during reprogramming, repressive modifications on histone H3 (e.g., H3K27me3) need to be removed and active modifications (e.g., H3K4me3) need to be established in the regulatory regions of these genes. If H3.3 replacement can facilitate the removal of H3 carrying repressive modifications in the donor nuclei, then these modifications should decrease along with the incorporation of maternal H3.3 prior to genome activation during reprogramming in SCNT embryos.
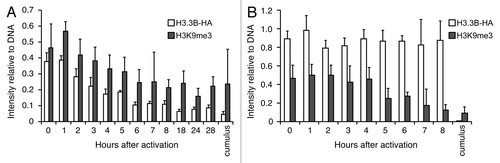
To address this hypothesis, we transferred H3.3B-HA cumulus cell nuclei into wild-type enucleated oocytes and monitored H3.3B-HA and H3 modifications by immunofluorescence at different time points in the nuclei of SCNT embryos post-activation. We observed that H3K4me3 levels began to increase at 5 h and peaked at 20 h post-activation, highly similar to the pattern observed for incorporation of maternal H3.3 into donor nuclei (). Conversely, the levels of H3K27me3 decreased in parallel with removal of donor nuclear H3.3 from the nuclei of SCNT embryos (before 20 h of activation) (). Knockdown of maternal H3.3 did not block the gain of H3K4me3 in the donor nuclei of SCNT embryos (), suggesting that this process is independent of maternal H3.3. Notably, the loss of donor nuclear H3K27me3 was impaired when maternal H3.3 was knocked down in SCNT embryos (), suggesting that loss of H3K27me3 in the donor nuclei of SCNT embryos is dependent upon the incorporation of maternal H3.3. We examined another repressive modification, H3K9me3, a hallmark of heterochromatin.Citation37 The loss of donor nuclear H3K9me3 was also concurrent with the loss of donor nucleus-derived H3.3 in the nuclei of SCNT embryos (); however, knockdown of maternal H3.3 did not block the loss of donor nuclear H3K9me3 (), indicating that this process is not associated with the incorporation of maternal H3.3.
Figure 1. Dynamics of H3.3 and histone modifications during reprogramming in SCNT embryos. (A) and (B) Dynamics of H3K4me3 in the nuclei of SCNT embryos. H3K4me3 levels in the donor nuclei increase from 5 h to 20 h after activation in SCNT embryos (Arrows indicate the transplanted cumulus nuclei). Data are represented as H3K4me3 fluorescence intensities relative to DNA at various time points, with error representing SD (n ≥ 5). (C) and (D) Dynamics of H3K27me3 in the nuclei of H3.3B-HA cumulus nuclear transfer embryos. The H3K27me3 is gradually lost in the donor nuclei of SCNT embryos after activation (Arrows indicate the transplanted cumulus nuclei). Data are represented as H3K27me3 fluorescence intensities relative to DNA at various time points, with error representing SD (n ≥ 5). Bar scale: 20 µm.
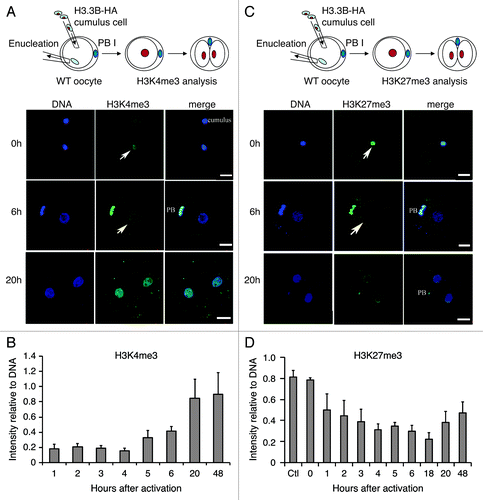
Figure 2. Dynamics of H3.3 and histone modifications during reprogramming in H3.3KD SCNT embryos. (A-C) Knockdown of maternal H3.3 inhibited the removal of donor H3.3B-HA, but did not alter the gain of the H3K4me3 in the donor nuclei of cumulus SCNT embryos. PB, the first polar body from H3.3B-HA wild-type oocytes. Data are represented as H3.3B-HA and H3K4me3 fluorescence intensities relative to DNA at various time points, with error representing SD (n ≥ 5). (D-F) Knockdown of maternal H3.3 in the oocytes impairs the loss of both donor cell-derived H3.3B-HA and H3K27me3 in SCNT embryos. Cumulus, H3.3B-HA wild-type cumulus cells. Data are represented as H3.3B-HA and H3K27me3 fluorescence intensities relative to DNA at various time points, with error representing SD (n ≥ 5). Bar scale: 20 µm.
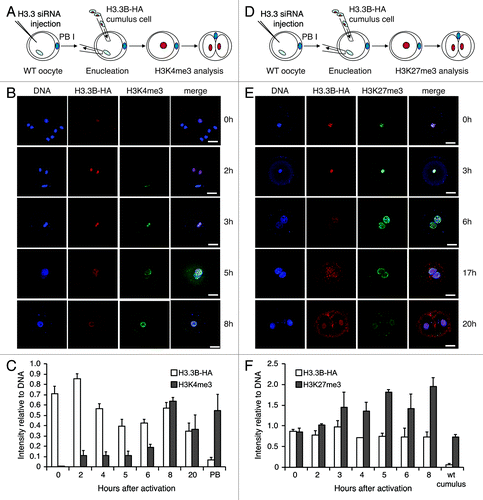
Figure 3. Dynamics of H3.3 and H3K9me3 during reprogramming in H3.3KD SCNT embryos. (A) Dynamics of donor cell-derived H3.3B-HA and H3K9me3 in the nuclei of H3.3B-HA cumulus SCNT embryos. The loss of donor cell-derived H3K9me3 is concurrent with the loss of donor cell-derived H3.3B-HA. (B) Dynamics of the donor cell-derived H3.3B-HA and H3K9me3 in the nuclei of H3.3KD SCNT embryos. The loss of donor cell-derived H3K9me3 is not altered when maternal H3.3 is knocked down. Cumulus: H3.3B-HA wild-type cumulus cells. Data are represented as H3.3B-HA and H3K9me3 fluorescence intensities relative to DNA at various time points, with error representing SD (n ≥ 5).
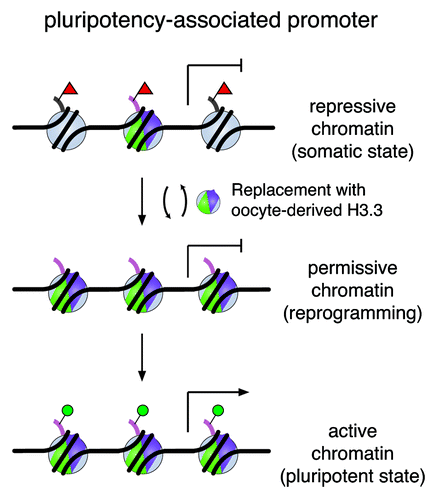
Overall, our data lead us to propose a working model by which the de novo synthesized H3.3 replaces histone H3 carrying repressive modifications in the donor nuclei of SCNT embryos, perhaps at specific loci that must be removed for successful gene reactivation. Consequently, this replacement would alter nucleosomal structure and enrich H3.3 in these loci. Through H3.3 replacement, the repressive chromatin state would be remodeled and transformed to a permissive state that is accessible to other reprogramming factors (). This mechanism would be in contrast to demethylases that actively remove repressive marks for gene activationCitation38 without directly altering nucleosomal structures, a process that may not depend on H3.3 during embryonic development.
Figure 4. A working model of H3.3 replacement in remodeling the chromatin for gene reactivation. A silenced pluripotency gene is marked with repressive modifications on histone H3 (e.g., H3K27me3, red triangles) on regulatory regions in somatic cells. De novo synthesized H3.3 protein (shown in green and purple) replaces histone H3 carrying modifications associated with repression during the reprogramming process. Consequently, this replacement alters nucleosomal structure and enriches H3.3 in these loci. Through H3.3 replacement, we speculate that the repressive chromatin is remodeled and transformed to a permissive state, which may be accessible to other regulatory factors for gene reactivation to establish a chromatin landscape poised for activation (e.g., H3K4me1, H3K4me3, H3K27ac, green circles) in the pluripotent state.
Concluding Remarks
The oocyte reprogramming process is characterized by the exchange of somatic proteins for oocyte proteins, extensive donor nuclear remodeling (including exchange of histone variants, histone modifications, and DNA methylation), and the reactivation of pluripotency genes.Citation11,Citation13 These events occur in an ordered manner and on a defined timescale, and are considered to be a deterministic process compared with the stochastic reprogramming process initiated by transcription factors.Citation11,Citation39 Our study demonstrates that the histone variant H3.3 is an essential maternal factor for oocyte reprogramming and is required for reactivation of many key pluripotency genes and the development of SCNT embryos. Our working model proposes that the maternally derived H3.3 protein replaces donor nucleus-derived H3 protein (both canonical H3 and variant H3.3), which carry repressive histone modifications (e.g., H3K27me3) at specific loci in the regulatory elements of genes. H3.3 replacement results in two consequences in the donor chromatin: (1) enrichment of H3.3 in these loci and (2) removal of repressive modifications in these loci. We hypothesize that chromatin remodeling of regulatory elements in the donor nucleus facilitates gene activation during the establishment of pluripotency. Our study provides a molecular model of chromatin remodeling events that are characteristic of reprogramming upon nuclear transfer. Future high-resolution ChIP-based studies of SCNT embryos will be required to validate our model of the relationship between H3.3 replacement and loss of histone repressive modifications. We anticipate that the role of H3.3 in somatic cell reprogramming is universal and is not specific to oocyte reprogramming. An induced reprogramming system based on transcription factors will be used to study the relationship between H3.3 replacement and histone modifications in the early stages of this reprogramming process.
Many outstanding questions remain. How is H3.3 replacement initiated, and what factors regulate this process? What are the signal(s) in the donor nucleus that determines replacement sites during reprogramming? Answering these questions will allow better understanding of the mechanism of the oocyte reprogramming process and lead to the design of optimal methods for conversion of somatic cells to the pluripotent state.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Acknowledgments
This work was supported by the Tri-Institutional Stem Cell Initiative (C.D.A., Z.R., and S.R.), in part by The Rockefeller University Fund (C.D.A.), Damon Runyon Cancer Research Foundation (L.A.B.), and the Rockefeller University Women and Science Initiative (L.A.B.).
References
- Boland MJ, Nazor KL, Loring JF. Epigenetic regulation of pluripotency and differentiation. Circ Res 2014; 115:311 - 24; http://dx.doi.org/10.1161/CIRCRESAHA.115.301517; PMID: 24989490
- Rivera RM, Ross JW. Epigenetics in fertilization and preimplantation embryo development. Prog Biophys Mol Biol 2013; 113:423 - 32; http://dx.doi.org/10.1016/j.pbiomolbio.2013.02.001; PMID: 23454467
- Wade PA, Kikyo N. Chromatin remodeling in nuclear cloning. Eur J Biochem 2002; 269:2284 - 7; http://dx.doi.org/10.1046/j.1432-1033.2002.02887.x; PMID: 11985609
- Tachibana M, Amato P, Sparman M, Gutierrez NM, Tippner-Hedges R, Ma H, Kang E, Fulati A, Lee HS, Sritanaudomchai H, et al. Human embryonic stem cells derived by somatic cell nuclear transfer. Cell 2013; 153:1228 - 38; http://dx.doi.org/10.1016/j.cell.2013.05.006; PMID: 23683578
- Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH. Viable offspring derived from fetal and adult mammalian cells. Nature 1997; 385:810 - 3; http://dx.doi.org/10.1038/385810a0; PMID: 9039911
- Wakayama T, Perry AC, Zuccotti M, Johnson KR, Yanagimachi R. Full-term development of mice from enucleated oocytes injected with cumulus cell nuclei. Nature 1998; 394:369 - 74; http://dx.doi.org/10.1038/28615; PMID: 9690471
- Gurdon JB, Partington GA, De Robertis EM. Injected nuclei in frog oocytes:RNA synthesis and protein exchange. J Embryol Exp Morphol 1976; 36:541 - 53; PMID: 1010978
- Jullien J, Astrand C, Halley-Stott RP, Garrett N, Gurdon JB. Characterization of somatic cell nuclear reprogramming by oocytes in which a linker histone is required for pluripotency gene reactivation. Proc Natl Acad Sci U S A 2010; 107:5483 - 8; http://dx.doi.org/10.1073/pnas.1000599107; PMID: 20212135
- Gao S, Chung YG, Parseghian MH, King GJ, Adashi EY, Latham KE. Rapid H1 linker histone transitions following fertilization or somatic cell nuclear transfer: evidence for a uniform developmental program in mice. Dev Biol 2004; 266:62 - 75; http://dx.doi.org/10.1016/j.ydbio.2003.10.003; PMID: 14729478
- Teranishi T, Tanaka M, Kimoto S, Ono Y, Miyakoshi K, Kono T, Yoshimura Y. Rapid replacement of somatic linker histones with the oocyte-specific linker histone H1foo in nuclear transfer. Dev Biol 2004; 266:76 - 86; http://dx.doi.org/10.1016/j.ydbio.2003.10.004; PMID: 14729479
- Jullien J, Pasque V, Halley-Stott RP, Miyamoto K, Gurdon JB. Mechanisms of nuclear reprogramming by eggs and oocytes: a deterministic process?. Nat Rev Mol Cell Biol 2011; 12:453 - 9; http://dx.doi.org/10.1038/nrm3140; PMID: 21697902
- Nashun B, Akiyama T, Suzuki MG, Aoki F. Dramatic replacement of histone variants during genome remodeling in nuclear-transferred embryos. Epigenetics 2011; 6:1489 - 97; http://dx.doi.org/10.4161/epi.6.12.18206; PMID: 22139579
- Wen D, Banaszynski LA, Liu Y, Geng F, Noh KM, Xiang J, Elemento O, Rosenwaks Z, Allis CD, Rafii S. Histone variant H3.3 is an essential maternal factor for oocyte reprogramming. Proc Natl Acad Sci U S A 2014; 111:7325 - 30; http://dx.doi.org/10.1073/pnas.1406389111; PMID: 24799717
- Jullien J, Astrand C, Szenker E, Garrett N, Almouzni G, Gurdon JB. HIRA dependent H3.3 deposition is required for transcriptional reprogramming following nuclear transfer to Xenopus oocytes. Epigenetics Chromatin 2012; 5:17; http://dx.doi.org/10.1186/1756-8935-5-17; PMID: 23102146
- Kang YK, Koo DB, Park JS, Choi YH, Chung AS, Lee KK, Han YM. Aberrant methylation of donor genome in cloned bovine embryos. Nat Genet 2001; 28:173 - 7; http://dx.doi.org/10.1038/88903; PMID: 11381267
- Chang G, Liu S, Wang F, Zhang Y, Kou Z, Chen D, Gao S. Differential methylation status of imprinted genes in nuclear transfer derived ES (NT-ES) cells. Genomics 2009; 93:112 - 9; http://dx.doi.org/10.1016/j.ygeno.2008.09.011; PMID: 18948186
- Lucifero D, Suzuki J, Bordignon V, Martel J, Vigneault C, Therrien J, Filion F, Smith LC, Trasler JM. Bovine SNRPN methylation imprint in oocytes and day 17 in vitro-produced and somatic cell nuclear transfer embryos. Biol Reprod 2006; 75:531 - 8; http://dx.doi.org/10.1095/biolreprod.106.051722; PMID: 16790688
- Brero A, Hao R, Schieker M, Wierer M, Wolf E, Cremer T, Zakhartchenko V. Reprogramming of active and repressive histone modifications following nuclear transfer with rabbit mesenchymal stem cells and adult fibroblasts. Cloning Stem Cells 2009; 11:319 - 29; http://dx.doi.org/10.1089/clo.2008.0083; PMID: 19508112
- Wu X, Li Y, Xue L, Wang L, Yue Y, Li K, Bou S, Li GP, Yu H. Multiple histone site epigenetic modifications in nuclear transfer and in vitro fertilized bovine embryos. Zygote 2011; 19:31 - 45; http://dx.doi.org/10.1017/S0967199410000328; PMID: 20609268
- Hanna JH, Saha K, Jaenisch R. Pluripotency and cellular reprogramming: facts, hypotheses, unresolved issues. Cell 2010; 143:508 - 25; http://dx.doi.org/10.1016/j.cell.2010.10.008; PMID: 21074044
- Yang X, Smith SL, Tian XC, Lewin HA, Renard JP, Wakayama T. Nuclear reprogramming of cloned embryos and its implications for therapeutic cloning. Nat Genet 2007; 39:295 - 302; http://dx.doi.org/10.1038/ng1973; PMID: 17325680
- Gao S, Gasparrini B, McGarry M, Ferrier T, Fletcher J, Harkness L, De Sousa P, Wilmut I. Germinal vesicle material is essential for nucleus remodeling after nuclear transfer. Biol Reprod 2002; 67:928 - 34; http://dx.doi.org/10.1095/biolreprod.102.004606; PMID: 12193404
- Ng RK, Gurdon JB. Epigenetic memory of an active gene state depends on histone H3.3 incorporation into chromatin in the absence of transcription. Nat Cell Biol 2008; 10:102 - 9; http://dx.doi.org/10.1038/ncb1674; PMID: 18066050
- Egli D, Birkhoff G, Eggan K. Mediators of reprogramming: transcription factors and transitions through mitosis. Nat Rev Mol Cell Biol 2008; 9:505 - 16; http://dx.doi.org/10.1038/nrm2439; PMID: 18568039
- Wellman SE, Casano PJ, Pilch DR, Marzluff WF, Sittman DB. Characterization of mouse H3.3-like histone genes. Gene 1987; 59:29 - 39; http://dx.doi.org/10.1016/0378-1119(87)90263-0; PMID: 2449379
- Frank D, Doenecke D, Albig W. Differential expression of human replacement and cell cycle dependent H3 histone genes. Gene 2003; 312:135 - 43; http://dx.doi.org/10.1016/S0378-1119(03)00609-7; PMID: 12909349
- Filipescu D, Szenker E, Almouzni G. Developmental roles of histone H3 variants and their chaperones. Trends Genet 2013; 29:630 - 40; http://dx.doi.org/10.1016/j.tig.2013.06.002; PMID: 23830582
- Loppin B, Bonnefoy E, Anselme C, Laurençon A, Karr TL, Couble P. The histone H3.3 chaperone HIRA is essential for chromatin assembly in the male pronucleus. Nature 2005; 437:1386 - 90; http://dx.doi.org/10.1038/nature04059; PMID: 16251970
- Santenard A, Ziegler-Birling C, Koch M, Tora L, Bannister AJ, Torres-Padilla ME. Heterochromatin formation in the mouse embryo requires critical residues of the histone variant H3.3. Nat Cell Biol 2010; 12:853 - 62; http://dx.doi.org/10.1038/ncb2089; PMID: 20676102
- Torres-Padilla ME, Bannister AJ, Hurd PJ, Kouzarides T, Zernicka-Goetz M. Dynamic distribution of the replacement histone variant H3.3 in the mouse oocyte and preimplantation embryos. Int J Dev Biol 2006; 50:455 - 61; PMID: 16586346
- van der Heijden GW, Dieker JW, Derijck AA, Muller S, Berden JH, Braat DD, van der Vlag J, de Boer P. Asymmetry in histone H3 variants and lysine methylation between paternal and maternal chromatin of the early mouse zygote. Mech Dev 2005; 122:1008 - 22; http://dx.doi.org/10.1016/j.mod.2005.04.009; PMID: 15922569
- Lin CJ, Conti M, Ramalho-Santos M. Histone variant H3.3 maintains a decondensed chromatin state essential for mouse preimplantation development. Development 2013; 140:3624 - 34; http://dx.doi.org/10.1242/dev.095513; PMID: 23903189
- Mtango NR, Potireddy S, Latham KE. Oocyte quality and maternal control of development. Int Rev Cell Mol Biol 2008; 268:223 - 90; http://dx.doi.org/10.1016/S1937-6448(08)00807-1; PMID: 18703408
- Banaszynski LA, Wen D, Dewell S, Whitcomb SJ, Lin M, Diaz N, Elsässer SJ, Chapgier A, Goldberg AD, Canaani E, et al. Hira-dependent histone H3.3 deposition facilitates PRC2 recruitment at developmental loci in ES cells. Cell 2013; 155:107 - 20; http://dx.doi.org/10.1016/j.cell.2013.08.061; PMID: 24074864
- Santos-Rosa H, Schneider R, Bannister AJ, Sherriff J, Bernstein BE, Emre NC, Schreiber SL, Mellor J, Kouzarides T. Active genes are tri-methylated at K4 of histone H3. Nature 2002; 419:407 - 11; http://dx.doi.org/10.1038/nature01080; PMID: 12353038
- Bogliotti YS, Ross PJ. Mechanisms of histone H3 lysine 27 trimethylation remodeling during early mammalian development. Epigenetics 2012; 7:976 - 81; http://dx.doi.org/10.4161/epi.21615; PMID: 22895114
- Peters AH, Mermoud JE, O’Carroll D, Pagani M, Schweizer D, Brockdorff N, Jenuwein T. Histone H3 lysine 9 methylation is an epigenetic imprint of facultative heterochromatin. Nat Genet 2002; 30:77 - 80; http://dx.doi.org/10.1038/ng789; PMID: 11740497
- Kooistra SM, Helin K. Molecular mechanisms and potential functions of histone demethylases. Nat Rev Mol Cell Biol 2012; 13:297 - 311; PMID: 22473470
- Yamanaka S. Elite and stochastic models for induced pluripotent stem cell generation. Nature 2009; 460:49 - 52; http://dx.doi.org/10.1038/nature08180; PMID: 19571877
