Abstract
Current therapies for advanced hepatocellular carcinoma (HCC) are marginally effective and exacerbate underlying liver disease. The ability of immunotherapy to elicit nontoxic, systemic, long-lived anti-tumor activity makes it particularly well-suited for use in the setting of HCC. While therapeutic benefit has been achieved in early clinical trials, the efficacy of immune-based therapies is limited by several unique properties of HCC, most notably the inherently tolerogenic character of the liver in both healthy and diseased (chronically-infected or tumor-bearing) states. Therapeutic regimens that both counteract these immunosuppressive mechanisms and amplify tumor-specific immunity are expected to profoundly improve clinical outcomes for HCC patients.
Introduction
Hepatocellular carcinoma (HCC) is one of the most common cancers in the world, with a global incidence of over 600,000 new cases per year. While the majority of cases are primarily in sub-Saharan Africa and Eastern Asia, it is also the fastest growing cause of cancer-related death of US males.Citation1 Within the past 30 y, the incidence and mortality rates for hepatocellular carcinoma (HCC) have tripled in the United States.Citation2 Racial differences in HCC incidence have been observed in the US, where Asians have higher rates than African Americans, who have higher rates than Caucasians.Citation2 The primary risk factors for developing HCC are cirrhosis (independent of its etiology), and chronic infection with hepatitis B virus (HBV) or hepatitis C virus (HCV). In the United States, it is estimated that chronic HCV infection is attributed to 47% of HCC cases, with an additional 15% associated with HBV.Citation3 HBV infection is endemic in South-East Asia and Sub-Saharan Africa, and there is a global pandemic of hepatitis C virus (HCV) infection. HCV infection, which increases the risk of developing HCC by approximately 17-fold, likely accounts for the increased incidence of HCC observed in several Western countries, where incidence has risen to 5–20/100,000 in Spain, Italy and Greece, and to 1–3.6/100,000 in the UK, Canada and the United States.Citation1 As diabetes, obesity and metabolic syndrome are also hypothesized risk factors, HCC is expected to become a progressively greater health problem in the near future.Citation4
Current therapies
Once diagnosed, HCC has a dismal prognosis. Small, localized tumors are potentially curable with surgery (resection and liver transplantation). Unfortunately, less than 20% of HCC patients are eligible for these procedures because most patients have advanced disease at diagnosis, have liver dysfunction limiting aggressive treatment, or have recurrent disease.Citation5 Local regional therapy is largely palliative and includes cryoablation, radiofrequency ablation (RFA), and transarterial embolization (TAE), in which obstruction of the hepatic artery induces subsequent tumor necrosis. HCC is notoriously resistant to chemotherapy and other systemic treatment modalities. The multi-targeted kinase inhibitor sorafenib, which improves survival by 2.3–2.8 mo, is the only systemic agent found to increase survival time in patients with advanced HCC and is currently the standard of care for these patients.Citation6,Citation7 Overall however, the median survival for patients with advanced stage, unresectable HCC is less than 1 y.Citation5
These reports underline the need for novel therapies for patients with this disease. A number of other molecularly targeted approaches, all of which target signaling pathways activated in HCC, are under investigation. These agents include bevacizumab, a vascular endothelial growth factor (VEGF) neutralizing antibody, sunitinib, a multi-targeted tyrosine kinase inhibitor (TKI), and erlotinib, an EGFR inhibitor.Citation7 However, the drug-metabolizing properties of the liver, in addition to elevated levels of multidrug resistance proteins expressed by HCC cells, likely contributes to the limited efficacy of chemotherapeutics and small molecule drugs in the treatment of HCC.Citation8 Moreover, these agents typically have intrinsic hepatotoxicity that may further compromise liver function. Immunotherapy represents an attractive alternative to these traditional therapies based on the sensitivity, specificity, and self-renewing capacity of the immune system.
Immunosuppressive Factors in HCC
Perhaps the most formidable barrier to immune-based therapy of HCC is the unique immunobiology of the liver. As described below, a plethora of regulatory mechanisms sustain the immunosuppressive milieu of the liver in both healthy and diseased (chronically-infected or tumor-bearing) states.
Inherent tolerogenicity of the liver
Blood from the arterial circulation and the intestines enter the liver, where toxins and gut-derived microbial products are captured and eliminated. To prevent aberrant immunity in response to continual pathogen exposure, the liver has evolved a unique and redundant system of immune regulation, as demonstrated by relatively low rates of liver allograft rejection and limited need for immune suppression post-transplant. Hepatocytes contribute to the liver’s inherent tolerogenicity by priming naïve T cells in the absence of costimulation, resulting in defective cytotoxicity and clonal deletion.Citation9,Citation10 Alternatively, naïve T cells that initially encounter antigen in the lymph nodes acquire full effector function, suggesting that effective immunotherapy in the setting of HCC must aim to avoid intrahepatic T cell priming.
Three distinct subsets of phagocytic cells in the liver clear foreign material from the blood and serve as tolerogenic antigen presenting cells (APC): (1) liver sinusoidal endothelial cells (LSEC), (2) Kupffer cells and (3) liver dendritic cells (DC). Liver sinusoidal endothelial cells line the hepatic vasculature and transport proteins from the circulation into the liver parenchyma. The specialized scavenger properties of LSEC also allows for the uptake, processing, and presentation of exogenous antigens on both major histocompatibility complex (MHC) class I and class II molecules. Cross-presentation of systemic antigen by these cells has been shown to induce antigen-specific CD8+ T cell tolerance through interactions between the inhibitory molecule B7-H1/PD-L1 expressed by LSEC and PD-1 on the T cells.Citation10 Importantly, high levels of exogenous interleukin-2 (IL-2) are capable of attenuating LSEC-mediated CD8+ T cell tolerance and restoring full T effector cell function.Citation11 While high dose IL-2 has been approved for use in melanoma and renal cell carcinoma, limitations of this therapy include severe side effects and expansion of Foxp3+ regulatory T cells (Treg).Citation12
Kupffer cells are tissue-resident macrophages that contribute to the immunosuppressive milieu of the liver through production of the anti-inflammatory molecules transforming growth factor-β (TGF-β), IL-10 and prostaglandin E2 (PGE2).Citation10 Kupffer cells also support systemic tolerance by eliminating high affinity antigen-specific CD8+ T cells that enter the liver.Citation13 Liver-resident DC represent another major subset of tolerogenic APC in the liver microenvironment. Priming of naïve CD4+ T cells by IL-10-secreting liver DC results in Th2 polarization, Treg induction, and poor antigen recall responses.Citation10 Additionally, myeloid DC precursors that enter the liver differentiate into IL-10-producing regulatory DC, indicating that in order to generate robust anti-HCC immunity, extrahepatic DC differentiation and maturation will be necessary.Citation14
Chronic HBV- and HCV-mediated immunosuppression
While persistent HBV or HCV infection can contribute to the development of HCC by driving chronic liver inflammation, the many immunosuppressive properties of chronic HBV or HCV infection have also been well-documented. For example, co-culture of the hepatitis B core antigen (HBcAg) with peripheral blood mononuclear cells (PBMC) from patients with chronic HBV infection induced elevated IL-10 production vs. PBMC from healthy controls.Citation15 Dysfunctional T cell responses to both virus-specific and unrelated antigens, characterized by impaired proliferation and IL-2 production, are also observed in chronic HBV-infected patients.Citation16 Furthermore, the frequencies of intrahepatic Treg in these patients correlates with viral load, suggesting that Treg accumulation in the chronically-infected liver may abrogate CD8+ T cell-mediated clearance of these viruses.Citation17
Chronic HCV infection has been found to negatively regulate both innate and adaptive arms of the immune system.Citation18 Monocyte-derived DC from HCV-infected patients, but not from healthy control donors, maintain an immature phenotype in response to maturation stimuli.Citation19 Because monocyte-derived DC from healthy donors cultured in the presence of HCV polyprotein also fail to respond to maturation stimuli, this inhibitory effect appears to be mediated through viral proteins.Citation20 Natural killer (NK) cells, which are abundant in the liver, are also susceptible to the tolerogenic properties of HCV-derived proteins. For example, high concentrations of HCV envelope protein E2 inhibit the effector function (IFN-γ production and cytotoxicity) of NK cells isolated from healthy donors.Citation21 At the T cell level, upregulation of the inhibitory receptors PD-1 and Tim-3, which correlates with T effector cell dysfunction, has been observed on both HCV-specific and HCV-nonspecific CD8+ T cells in the circulation and livers of patients with chronic HCV infection vs. normal controls.Citation22,Citation23 Partial restoration of T cell proliferation and IFN-γ secretion was achieved ex vivo by inhibiting the binding of PD-1 and Tim-3 to their respective ligands (B7-H1 and Gal-9). Importantly, a recent report has demonstrated that prolonged administration of IFN-α, a standard therapy for persistent HCV infection, promoted telomere loss in naïve T cells.Citation24 Given the correlation between shortened T cell telomeres and terminal differentiation (characterized by diminished proliferative potential), IFN-α-induced T cell “exhaustion” likely represents a significant barrier for immunotherapy in HCV-infected patients.Citation25
Mechanisms of HCC immune escape
In response to the accumulating literature supporting the ability of tumors to subvert an immune attack, Hanahan and Weinberg have recently updated their “Hallmarks of Cancer” to include tumor immune evasion.Citation26 HCC is not unlike cancers of other etiologies in that numerous regulatory mechanisms involving nearly all cellular subsets of the immune system contribute to tumor development and progression. Compared with healthy controls, the peripheral blood of HCC patients contain reduced frequencies of myeloid DC, which exhibit impaired IL-12 production and limited allo-stimulatory activity.Citation27 Diminished NK cell frequencies and functional impairment has also been described in HCC patients.Citation28 Moreover, NK cell dysfunction in these patients may be attributable to an expanded population of myeloid-derived suppressor cells (MDSC), which have been recently shown to inhibit NK cell activity through a cell contact-dependent mechanism.Citation29
A type-1 polarized tumor-specific T cell response is an essential component of robust anti-tumor immunity. Budhu and colleagues have demonstrated that liver-infiltrating lymphocytes in HCC patients exhibit a type-2-skewed anti-inflammatory profile that correlates with metastatic potential.Citation30 As in both the healthy liver and chronically HBV/HCV-infected liver, PD-1-B7-H1 interactions play an important role in maintaining immune tolerance in the setting of HCC. In individuals with persistent HBV infection, PD-1 expression by peripheral blood CD8+ cytotoxic T lymphocytes (CTL) is upregulated with disease progression from cirrhosis to HCC.Citation31 Aberrantly-activated monocytes that are enriched in HCC lesions have been shown to express high levels of the PD-1 ligand B7-H1 through a mechanism involving autocrine TNF-α and IL-10 production.Citation32 Notably, B7-H1 neutralization enhanced human PD-1+CD8+ T cell survival in vitro and restored tumor-specific T cell immunity in a tumor xenograft model.Citation31 Finally, the induction, expansion, and/or recruitment of Treg in the HCC tumor microenvironment are indisputable barriers to robust anti-tumor immunity, as intratumoral Treg density is associated with poor clinical prognosis.Citation31 Maintenance of an expanded pool of Treg in HCC patients is accomplished in part by MDSC, which have been shown to promote the conversion of naïve CD4+ T cells from the peripheral blood of these patients into Foxp3+ Treg.Citation33
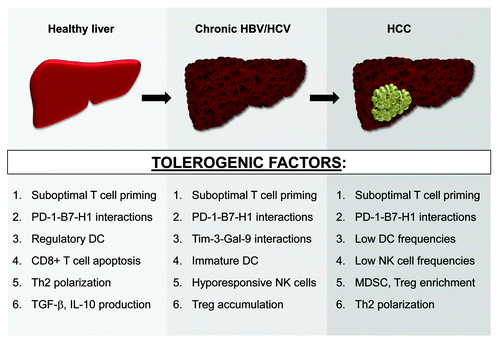
In summary, the liver is comprised of a dynamic network of specialized cell subsets that maintain immune privilege through redundant regulatory mechanisms. Three distinct liver microenvironments, varying with disease state and all polarized toward negative immune regulation, have been characterized (). These numerous tolerogenic factors may accumulate during HBV/HCV-mediated hepatocarcinogenesis and coincide in advanced HCC lesions, facilitating an aggressive and effective counterattack to anti-HCC immunity. Future studies will be needed to address this notion.
Figure 1. Evolving liver immunobiology during HCC development. Numerous tolerogenic factors, many of which are listed here, support immunoregulation in both the steady-state and diseased (chronically-infected or tumor-bearing) liver. These immunosuppressive mechanisms likely accumulate during HBV/HCV-mediated hepatocarcinogenesis and coexist in patients with advanced HCC lesions. See text for all associated references.
Immunotherapy of HCC
While HCC is not generally considered an “immunogenic” tumor, patients whose tumors contain lymphocytic infiltrates show longer survival and lower risk of recurrence.Citation34 In the past 15 y, several tumor-associated antigens for HCC have been identified, and a measure of success has been achieved in early clinical trials.
HCC-specific antigens
AFP is an oncofetal antigen and is the most abundant serum protein in the fetus.Citation35 At birth, levels drop to 30–100 μg/ml and the adult level of AFP is 1–3 ng/ml. It is produced at high levels by the visceral endoderm of the yolk sac and fetal liver, and is transcriptionally repressed shortly after birth. Forty to 80% of HCC and germ cell tumors express AFP, and serum assays play an important role in diagnosis and monitoring responses to treatment; levels > 20 ng/ml are generally considered abnormal, and > 200 ng/ml are specific for HCC in patients with cirrhosis.Citation5 Human AFP is translated as a 609 residue polypeptide that is cleaved to yield a 590 amino acid secreted protein. The function of AFP is unknown. It has been hypothesized to play a role in the transport of serum components, including fatty acids, steroids, and heavy metals.Citation36 There have also been reports of an immunoregulatory role of AFP.Citation37
Although high levels of soluble AFP present during development could have a broad impact on the developing T cell repertoire, including deletion of high affinity T cells, murine studies have demonstrated that murine AFP can serve as a tumor rejection antigen.Citation38 Studies in HCC patients indicate that circulating AFP-specific T cells can be activated ex vivo and can recognize tumor despite high circulating serum levels of this antigen.Citation39-Citation41 Polyclonal AFP-specific T cells can also be detected in the livers of chronically infected HCV patients and HCC patients.Citation42 Furthermore, elimination of Treg can unmask AFP-specific T cells in HCC patients.Citation43 Importantly, AFP expression in HCC is associated with increased tumor proliferation, apoptosis resistance, and it is expressed in CD45-CD90+ HCC cancer stem cells, supporting its targeting as a biologically relevant tumor associated antigen.Citation36,Citation44
Among additional tumor antigens implicated in anti-HCC immunity are the cancer-testes antigens, such as those of the highly homologous MAGE-A family, which have demonstrated considerable immunogenicity in several clinical trials. MAGE-A3, for example, has been found to be overexpressed in 24–70% of HCC.Citation45 Glypican-3 (GPC3), a cell surface heparin sulfate proteoglycan, is expressed by 84% of HCC and has been shown to be immunogenic in murine models and human cell culture.Citation46 Because the frequency of GPC3-specific T cells is low in the peripheral blood of HCC patients, optimized costimulatory conditions may be required for ex vivo expansion of these cells.Citation47 NY-ESO-1 is an additional cancer/testes antigen expressed in HCC. Spontaneous CD8+ T cell and antibody responses have been detected in HCC patients, supporting the use of NY-ESO-1 as an immunotherapy target in HCC.Citation45 Wilms' tumor 1 (WT-1) has also been identified as a tumor-associated antigen in HCC.Citation48 Importantly, elevated WT-1 expression is also found in hepatocytes from patients with fibrosis and chronic hepatitis, two common conditions preceding HCC development.Citation49 Finally, because telomerase activity is upregulated during hepatocarcinogenesis, telomerase-based vaccines have been tested in HCC patients, although the initial trial results did not show evidence of immune or clinical responses.Citation45,Citation50
Current immunotherapy of HCC
Results from several clinical trials demonstrate that immune-based therapy can improve outcomes for patients with HCC.Citation51 In one large trial, 150 patients were randomized to receive either IL-2 and anti-CD3-activated PBMC or observation post curative resection.Citation52 There were statistically significant improvements in time to recurrence as well as recurrence-free survival (overall survival was improved, p = 0.09). The largest trial testing infusion of APC included 31 HCC patients who received DC pulsed with autologous tumor lysate.Citation53 Overall, there were 14 partial responses and 17 disease stabilizations, and patients had an improved 1 y survival (63% vs. 10%; p = 0.038). These results support the notion of DC-based immunotherapy for HCC. Murine models also support immunotherapy, most recently demonstrating that DC in combination with IL-12 in an adjuvant setting activates T and NK cells and reduces HCC recurrence.Citation54
Future Immunotherapeutic Strategies
New approaches
In light of the numerous regulatory mechanisms that HCC tumors employ to evade an immune attack, a major goal of novel immunotherapies in HCC will be to antagonize these regulatory circuits. The CTLA-4 blocking antibody ipilimumab is capable of inducing durable clinical responses and objective response rates of approximately 10% in metastatic melanoma.Citation55 In the setting of HCC, ex vivo treatment of CD8+ T cells isolated from HCC patients with CTLA-4 blocking antibodies resulted in an expanded antigen-specific T cell repertoire, suggesting that ipilimumab likely possesses high potential for the treatment of HCC.Citation56 Antibody-mediated blockade of PD-1 has demonstrated therapeutic benefit in the setting of refractory solid tumors, while inhibition of Tim-3 signaling in pre-clinical models has been shown to restore anti-tumor T cell activity.Citation57,Citation58 Moreover, Treg-depleting reagents, such as denileukin diftitox (Ontak), that target the constitutively-expressed molecule CD25, have been shown to effectively eliminate circulating Tregs without coordinate depletion of activated, CD25-expressing T effector cells.Citation59 Another strategy to overcome tumor-mediated immunosuppression involves the direct reactivation of hyporesponsive tumor-specific T cells through provision of T cell growth factors (IL-15, IL-7) or costimulatory agonists (anti-4–1BB, anti-OX40).Citation60,Citation61 Although these reagents have been tested extensively in other prevalent cancers, their therapeutic potential in HCC has unfortunately yet to be explored.
Treatment modalities that amplify tumor homing and penetration of T effector cells are also anticipated to have significant therapeutic potential in HCC, given the well-established correlation between T cell infiltration of HCC lesions and overall survival.Citation34 These strategies can be divided into two general categories: (1) normalization of the tumor vasculature and (2) chemokine and adhesion molecule upregulation.
The vasculature of highly angiogenic cancers, such as HCC, is characterized by disorganized, tortuous vessels that restrict the infiltration of nutrients, oxygen, therapeutic drugs and tumor-primed T cells.Citation62 In pre-clinical models, treatment-induced remodeling of the tumor vasculature has restored T cell infiltration of tumors and demonstrated anti-tumor efficacy.Citation63 Currently however, small molecule drugs and monoclonal antibodies targeting VEGF and its receptors, including sorafenib and bevacizumab, have demonstrated limited therapeutic efficacy in HCC clinical trials.Citation6,Citation7 Immunotherapy targeting angiogenesis and/or the tumor vasculature represents a feasible alternative based on the potential to generate long-lived anti-vascular T cell responses. This notion is supported by pre-clinical data from Niethammer and colleagues, who show that an anti-VEGFR2 vaccine suppresses angiogenesis and tumor dissemination through a T cell-dependent process.Citation64 Immune-based anti-angiogenic agents may therefore prove useful in the setting of HCC.
Circulating T cells extravasate into peripheral tissues through recognition of adhesion molecules, including ICAM-1 and VCAM-1, which are upregulated by vascular endothelial cells in response to inflammatory conditions. In the healthy liver, VLA-4-expressing Th1 cells are preferentially recruited via VCAM-1 on the sinusoidal venules.Citation65 VCAM-1 also plays an important role in the sequestration of activated T cells in the liver microvasculature.Citation66 These data suggest that strategies to upregulate VCAM-1 expression on the tumor vasculature will likely augment pre-existing or therapy-induced anti-HCC T cell activity. The importance of pro-inflammatory chemokine molecules in HCC-specific T cell immunity has also been demonstrated, as high levels of the IFN-γ-inducible chemokines CXCL9/Mig and CXCL10/IP-10 correlated with HCC tumor enrichment of CD8+ T cells.Citation67 Although this pattern of chemokine expression correlates with favorable prognosis of patients with uterine/cervical cancers, it is currently unclear whether the same is true for HCC patients.Citation68 Therapeutic reagents that induce chemokine and adhesion molecule expression through vascular activation are therefore an attractive approach for the immune-based treatment of HCC.
Combinational regimens
Although single agent modalities have demonstrated some efficacy in the clinic, immunotherapy in combination with conventional treatments (chemo-immunotherapy) or other immunotherapies is expected to elicit synergistic anti-tumor activity. Several chemotherapeutic agents, including adriamycin, cisplatin and gemcitabine are commonly used for ablative treatment of HCC. A number of recent studies have more closely examined the immunological impact of some of these agents in various tumor types and the modes of death induced by them.Citation69 For example, gemcitabine-induced tumor apoptosis can promote tumor antigen cross-presentation by DC and resultant T cell responses.Citation70 Non-chemotherapeutic ablative therapies for HCC, including transarterial embolization (TAE) and radiofrequency ablation (RFA), can induce immunogenic tumor cell death as well. Embolized patients tested before and following treatment increased the frequency of AFP-specific CD4+ T cells, while HCC patients tested after RFA demonstrated improved T cell responses to autologous tumor and had increased circulating NK cell frequencies, likely due to the immunogenicity of released antigen.Citation71,Citation72 Early evidence suggests that immunotherapy will be most effective during or shortly after ablative therapy, when tumor cells are dying and an active immune response has commenced. In HCC, combined therapy of TAE with intratumoral dendritic cell infusion induced higher frequencies of AFP-specific T cells compared with TAE alone.Citation73 Therefore, there are many opportunities for combining immunotherapy with an immunogenic form of HCC ablation to improve antitumor responses. The rational choice of the ablation approach may provide an improved and more immunogenic setting for subsequent immunization, and potentially a “priming” event after which a vaccine would be a “boost” in a combination therapy.
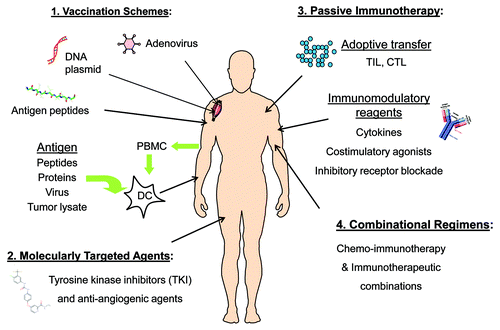
Despite the observation that long-term IFN-α therapy in patients with advanced hepatitis C does not reduce the incidence of HCC, IFN-α administration in combination with tumor-specific vaccination, which elicited synergistic anti-tumor T cell activity in melanoma patients, could be a viable therapeutic option in the setting of HCC.Citation74,Citation75 Many opportunities clearly exist for therapeutic intervention in advanced HCC, including both single agent modalities and combinational regimens (). Optimal therapeutic strategies in HCC will presumably involve combinations of immunotherapy that engage multiple immune effector mechanisms, such as vaccines and T cell immunomodulators, as well as immunotherapy supplemented with molecularly targeted inhibitors of tumor signaling pathways.Citation76
Figure 2. Future therapeutic strategies for HCC. (1) Several vaccine approaches have been tested in HCC, including strategies to target AFP and other antigens by DNA, virus, peptide and DC-based vaccines. (2) Sorafenib has been licensed for HCC and other next-generation TKIs are in clinical testing, while bevacizumab (anti-VEGF) is under investigation in HCC as well. (3) Immunomodulatory agents, including those to release immune suppression and boost T cell function, are promising. While more technically challenging, adoptive transfer of antigen-specific T cells, such as tumor-infiltrating lymphocytes (TIL), are nearing clinical translation at more sites around the globe. (4) Rationally designed combinations of standard-of-care ablation approaches (i.e., chemotherapy, RFA, TAE) with immunotherapy strategies will likely work synergistically to improve clinical outcomes for HCC patients.
Conclusions and Additional Considerations
As described above, the distinctly immunosuppressive milieu of both the steady-state and diseased liver serves as a considerable obstacle to effective HCC immunotherapy. Beyond the tolerogenic microenvironment, however, there are additional properties unique to HCC that may further impede the successful implementation of immunotherapy. First, many investigators have observed extensive patient-to-patient heterogeneity in the molecular and cellular makeup of HCC tumors, and accumulating evidence suggests that this is likely a result of the multiple etiological factors involved in hepatocarcinogenesis (i.e., HBV/HCV infection, chronic alcohol consumption, metabolic syndrome).Citation77 For example, preferential upregulation of NK cell-associated KIRs (killer cell immunoglobulin-like receptors) has been reported in HCV-infected (and not HBV-infected) HCC patients.Citation78 Due to the inhibitory nature of KIR molecules, HCV-related HCC tumors may be particularly resistant to NK cell-mediated cytotoxicity compared with HCC lesions of alternative etiologies. Additionally, Miroux and colleagues have suggested that because negligible frequencies of Tregs are found in alcohol-related HCC patients, Treg recruitment to the liver may be specifically linked to HCV infection.Citation17 Although more research must be done in this area, differing etiologies likely have a direct impact on disease course and treatment outcome, and therapeutic regimens may need to be tailored to individual HCC patients based on their distinct etiological factor(s). Lastly, in the minority of HCC patients who are eligible for and receive liver transplantation, recurrent disease develops in up to 20% of these patients.Citation5 It has been hypothesized that immunosuppressant agents directly contribute to post-transplant HCC recurrence by promoting tumor immune evasion.Citation79 The choice of immunosuppressive drug based on their differential modes of action could therefore prove important in the prevention of HCC recurrence following liver transplantation.Citation80
While early-phase clinical trials serve as proof-of-concept that immunotherapy of HCC is safe, feasible, and moderately effective, future trials must take into account several unique properties of HCC that may have limited the success of previous trials. Inclusion criteria should be tightened to reduce heterogeneity among patients, etiologic factors and liver function. Blood and tissue samples need to routinely be banked in order to help identify patient subsets and biomarkers of response (both predictive and prognostic). Finally, therapeutic agents that are capable of reversing the immunosuppressive nature of HCC tumors, administered alone or more likely in combination with other modalities, will be critical to optimizing clinical outcomes for HCC patients.
| Abbreviations: | ||
| HCC | = | hepatocellular carcinoma |
| HBV | = | hepatitis B virus |
| HCV | = | hepatitis C virus |
| RFA | = | radiofrequency ablation |
| TAE | = | transarterial embolization |
| VEGF | = | vascular endothelial growth factor |
| TKI | = | tyrosine kinase inhibitor |
| LSEC | = | liver sinusoidal endothelial cell |
| APC | = | antigen presenting cell |
| DC | = | dendritic cell |
| PBMC | = | peripheral blood mononuclear cells |
| MHC | = | major histocompatibility complex |
| IL | = | interleukin |
| IFN | = | interferon |
| NK | = | natural killer |
| MDSC | = | myeloid-derived suppressor cell |
| Treg | = | regulatory T cell |
| CTL | = | cytotoxic T lymphocytes |
| AFP | = | alpha-fetoprotein |
| GPC3 | = | glypican-3 |
Acknowledgments
This study was supported by research funding from the University of Pittsburgh Cancer Institute, NCI RO1 CA 138635 (LHB), and was supported in part by the UPCI CCSG award P30CA047904.
Disclosure of Potential Conflicts of Interest
ADP: none to declare. LHB is co-inventor of patents covering aspects of AFP as a target for T cell-mediated anti-HCC immunity.
References
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007; 132:2557 - 76; http://dx.doi.org/10.1053/j.gastro.2007.04.061; PMID: 17570226
- Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol 2009; 27:1485 - 91; http://dx.doi.org/10.1200/JCO.2008.20.7753; PMID: 19224838
- Di Bisceglie AM, Lyra AC, Schwartz M, Reddy RK, Martin P, Gores G, et al. Hepatitis C-related hepatocellular carcinoma in the United States: influence of ethnic status. Am J Gastroenterol 2003; 98:2060 - 3; http://dx.doi.org/10.1016/S0002-9270(03)00552-5; PMID: 14499788
- Nordenstedt H, White DL, El-Serag HB. The changing pattern of epidemiology in hepatocellular carcinoma. Dig Liver Dis 2010; 42:Suppl 3 S206 - 14; http://dx.doi.org/10.1016/S1590-8658(10)60507-5; PMID: 20547305
- El-Serag HB, Marrero JA, Rudolph L, Reddy KR. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology 2008; 134:1752 - 63; http://dx.doi.org/10.1053/j.gastro.2008.02.090; PMID: 18471552
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359:378 - 90; http://dx.doi.org/10.1056/NEJMoa0708857; PMID: 18650514
- Siegel AB, Olsen SK, Magun A, Brown RS Jr. Sorafenib: where do we go from here?. Hepatology 2010; 52:360 - 9; http://dx.doi.org/10.1002/hep.23633; PMID: 20578152
- Song R, Ikeguchi M, Zhou G, Kuo MT. Identification and characterization of a hepatoma cell-specific enhancer in the mouse multidrug resistance mdr1b promoter. J Biol Chem 1995; 270:25468 - 74; http://dx.doi.org/10.1074/jbc.270.43.25468; PMID: 7592715
- Bowen DG, Zen M, Holz L, Davis T, McCaughan GW, Bertolino P. The site of primary T cell activation is a determinant of the balance between intrahepatic tolerance and immunity. J Clin Invest 2004; 114:701 - 12; PMID: 15343389
- Thomson AW, Knolle PA. Antigen-presenting cell function in the tolerogenic liver environment. Nat Rev Immunol 2010; 10:753 - 66; http://dx.doi.org/10.1038/nri2858; PMID: 20972472
- Schurich A, Berg M, Stabenow D, Bottcher J, Kern M, Schild HJ, et al. Dynamic regulation of CD8 T cell tolerance induction by liver sinusoidal endothelial cells. J Immunol 2010; 184:4107 - 14; http://dx.doi.org/10.4049/jimmunol.0902580; PMID: 20212092
- Ahmadzadeh M, Rosenberg SA. IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients. Blood 2006; 107:2409 - 14; http://dx.doi.org/10.1182/blood-2005-06-2399; PMID: 16304057
- Kuniyasu Y, Marfani SM, Inayat IB, Sheikh SZ, Mehal WZ. Kupffer cells required for high affinity peptide-induced deletion, not retention, of activated CD8+ T cells by mouse liver. Hepatology 2004; 39:1017 - 27; http://dx.doi.org/10.1002/hep.20153; PMID: 15057906
- Xia S, Guo Z, Xu X, Yi H, Wang Q, Cao X. Hepatic microenvironment programs hematopoietic progenitor differentiation into regulatory dendritic cells, maintaining liver tolerance. Blood 2008; 112:3175 - 85; http://dx.doi.org/10.1182/blood-2008-05-159921; PMID: 18669892
- Hyodo N, Nakamura I, Imawari M. Hepatitis B core antigen stimulates interleukin-10 secretion by both T cells and monocytes from peripheral blood of patients with chronic hepatitis B virus infection. Clin Exp Immunol 2004; 135:462 - 6; http://dx.doi.org/10.1111/j.1365-2249.2003.02376.x; PMID: 15008979
- Das A, Hoare M, Davies N, Lopes AR, Dunn C, Kennedy PT, et al. Functional skewing of the global CD8 T cell population in chronic hepatitis B virus infection. J Exp Med 2008; 205:2111 - 24; http://dx.doi.org/10.1084/jem.20072076; PMID: 18695005
- Miroux C, Vausselin T, Delhem N. Regulatory T cells in HBV and HCV liver diseases: implication of regulatory T lymphocytes in the control of immune response. Expert Opin Biol Ther 2010; 10:1563 - 72; http://dx.doi.org/10.1517/14712598.2010.529125; PMID: 20932226
- Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol 2005; 5:215 - 29; http://dx.doi.org/10.1038/nri1573; PMID: 15738952
- Auffermann-Gretzinger S, Keeffe EB, Levy S. Impaired dendritic cell maturation in patients with chronic, but not resolved, hepatitis C virus infection. Blood 2001; 97:3171 - 6; http://dx.doi.org/10.1182/blood.V97.10.3171; PMID: 11342445
- Saito K, Ait-Goughoulte M, Truscott SM, Meyer K, Blazevic A, Abate G, et al. Hepatitis C virus inhibits cell surface expression of HLA-DR, prevents dendritic cell maturation, and induces interleukin-10 production. J Virol 2008; 82:3320 - 8; http://dx.doi.org/10.1128/JVI.02547-07; PMID: 18216090
- Tseng CT, Klimpel GR. Binding of the hepatitis C virus envelope protein E2 to CD81 inhibits natural killer cell functions. J Exp Med 2002; 195:43 - 9; http://dx.doi.org/10.1084/jem.20011145; PMID: 11781364
- Golden-Mason L, Palmer B, Klarquist J, Mengshol JA, Castelblanco N, Rosen HR. Upregulation of PD-1 expression on circulating and intrahepatic hepatitis C virus-specific CD8+ T cells associated with reversible immune dysfunction. J Virol 2007; 81:9249 - 58; http://dx.doi.org/10.1128/JVI.00409-07; PMID: 17567698
- Golden-Mason L, Palmer BE, Kassam N, Townshend-Bulson L, Livingston S, McMahon BJ, et al. Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4+ and CD8+ T cells. J Virol 2009; 83:9122 - 30; http://dx.doi.org/10.1128/JVI.00639-09; PMID: 19587053
- O'Bryan JM, Potts JA, Bonkovsky HL, Mathew A, Rothman AL. Extended interferon-alpha therapy accelerates telomere length loss in human peripheral blood T lymphocytes. PLoS ONE 2011; 6:e20922; http://dx.doi.org/10.1371/journal.pone.0020922; PMID: 21829595
- Hinrichs CS, Borman ZA, Gattinoni L, Yu Z, Burns WR, Huang J, et al. Human effector CD8+ T cells derived from naive rather than memory subsets possess superior traits for adoptive immunotherapy. Blood 2011; 117:808 - 14; http://dx.doi.org/10.1182/blood-2010-05-286286; PMID: 20971955
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646 - 74; http://dx.doi.org/10.1016/j.cell.2011.02.013; PMID: 21376230
- Ormandy LA, Farber A, Cantz T, Petrykowska S, Wedemeyer H, Horning M, et al. Direct ex vivo analysis of dendritic cells in patients with hepatocellular carcinoma. World J Gastroenterol 2006; 12:3275 - 82; PMID: 16718852
- Cai L, Zhang Z, Zhou L, Wang H, Fu J, Zhang S, et al. Functional impairment in circulating and intrahepatic NK cells and relative mechanism in hepatocellular carcinoma patients. Clin Immunol 2008; 129:428 - 37; http://dx.doi.org/10.1016/j.clim.2008.08.012; PMID: 18824414
- Hoechst B, Voigtlaender T, Ormandy L, Gamrekelashvili J, Zhao F, Wedemeyer H, et al. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology 2009; 50:799 - 807; http://dx.doi.org/10.1002/hep.23054; PMID: 19551844
- Budhu A, Forgues M, Ye QH, Jia HL, He P, Zanetti KA, et al. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell 2006; 10:99 - 111; http://dx.doi.org/10.1016/j.ccr.2006.06.016; PMID: 16904609
- Shirabe K, Motomura T, Muto J, Toshima T, Matono R, Mano Y, et al. Tumor-infiltrating lymphocytes and hepatocellular carcinoma: pathology and clinical management. Int J Clin Oncol 2010; 15:552 - 8; http://dx.doi.org/10.1007/s10147-010-0131-0; PMID: 20963618
- Chen J, Li G, Meng H, Fan Y, Song Y, Wang S, et al. Upregulation of B7-H1 expression is associated with macrophage infiltration in hepatocellular carcinomas. Cancer Immunol Immunother 2011; in press; http://dx.doi.org/10.1007/s00262-011-1094-3; PMID: 21853301
- Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Kruger C, Manns MP, et al. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology 2008; 135:234 - 43; http://dx.doi.org/10.1053/j.gastro.2008.03.020; PMID: 18485901
- Wada Y, Nakashima O, Kutami R, Yamamoto O, Kojiro M. Clinicopathological study on hepatocellular carcinoma with lymphocytic infiltration. Hepatology 1998; 27:407 - 14; http://dx.doi.org/10.1002/hep.510270214; PMID: 9462638
- Mizejewski GJ. Alpha-fetoprotein structure and function: relevance to isoforms, epitopes, and conformational variants. Exp Biol Med (Maywood) 2001; 226:377 - 408; PMID: 11393167
- Mizejewski GJ. Biological role of alpha-fetoprotein in cancer: prospects for anticancer therapy. Expert Rev Anticancer Ther 2002; 2:709 - 35; http://dx.doi.org/10.1586/14737140.2.6.709; PMID: 12503217
- Um SH, Mulhall C, Alisa A, Ives AR, Karani J, Williams R, et al. Alpha-fetoprotein impairs APC function and induces their apoptosis. J Immunol 2004; 173:1772 - 8; PMID: 15265907
- Meng WS, Butterfield LH, Ribas A, Dissette VB, Heller JB, Miranda GA, et al. alpha-Fetoprotein-specific tumor immunity induced by plasmid prime-adenovirus boost genetic vaccination. Cancer Res 2001; 61:8782 - 6; PMID: 11751399
- Butterfield LH, Ribas A, Meng WS, Dissette VB, Amarnani S, Vu HT, et al. T-cell responses to HLA-A*0201 immunodominant peptides derived from alpha-fetoprotein in patients with hepatocellular cancer. Clin Cancer Res 2003; 9:5902 - 8; PMID: 14676113
- Butterfield LH, Ribas A, Potter DM, Economou JS. Spontaneous and vaccine induced AFP-specific T cell phenotypes in subjects with AFP-positive hepatocellular cancer. Cancer Immunol Immunother 2007; 56:1931 - 43; http://dx.doi.org/10.1007/s00262-007-0337-9; PMID: 17522860
- Evdokimova VN, Liu Y, Potter DM, Butterfield LH. AFP-specific CD4+ helper T-cell responses in healthy donors and HCC patients. J Immunother 2007; 30:425 - 37; http://dx.doi.org/10.1097/CJI.0b013e31802fd8e2; PMID: 17457217
- Thimme R, Neagu M, Boettler T, Neumann-Haefelin C, Kersting N, Geissler M, et al. Comprehensive analysis of the alpha-fetoprotein-specific CD8+ T cell responses in patients with hepatocellular carcinoma. Hepatology 2008; 48:1821 - 33; http://dx.doi.org/10.1002/hep.22535; PMID: 19003875
- Greten TF, Ormandy LA, Fikuart A, Hochst B, Henschen S, Horning M, et al. Low-dose cyclophosphamide treatment impairs regulatory T cells and unmasks AFP-specific CD4+ T-cell responses in patients with advanced HCC. J Immunother 2010; 33:211 - 8; http://dx.doi.org/10.1097/CJI.0b013e3181bb499f; PMID: 20139774
- Yang ZF, Ho DW, Ng MN, Lau CK, Yu WC, Ngai P, et al. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell 2008; 13:153 - 66; http://dx.doi.org/10.1016/j.ccr.2008.01.013; PMID: 18242515
- Greten TF, Manns MP, Korangy F. Immunotherapy of hepatocellular carcinoma. J Hepatol 2006; 45:868 - 78; http://dx.doi.org/10.1016/j.jhep.2006.09.004; PMID: 17046096
- Ho M, Kim H. Glypican-3: a new target for cancer immunotherapy. Eur J Cancer 2011; 47:333 - 8; http://dx.doi.org/10.1016/j.ejca.2010.10.024; PMID: 21112773
- Xu Y, Li H, Gao RL, Adeyemo O, Itkin M, Kaplan DE. Expansion of interferon-gamma-producing multifunctional CD4+ T-cells and dysfunctional CD8+ T-cells by glypican-3 peptide library in hepatocellular carcinoma patients. Clin Immunol 2011; 139:302 - 13; http://dx.doi.org/10.1016/j.clim.2011.02.014; PMID: 21419713
- Sera T, Hiasa Y, Mashiba T, Tokumoto Y, Hirooka M, Konishi I, et al. Wilms' tumour 1 gene expression is increased in hepatocellular carcinoma and associated with poor prognosis. Eur J Cancer 2008; 44:600 - 8; http://dx.doi.org/10.1016/j.ejca.2008.01.008; PMID: 18255279
- Berasain C, Herrero JI, Garcia-Trevijano ER, Avila MA, Esteban JI, Mato JM, et al. Expression of Wilms' tumor suppressor in the liver with cirrhosis: relation to hepatocyte nuclear factor 4 and hepatocellular function. Hepatology 2003; 38:148 - 57; http://dx.doi.org/10.1053/jhep.2003.50269; PMID: 12829997
- Greten TF, Forner A, Korangy F, N'Kontchou G, Barget N, Ayuso C, et al. A phase II open label trial evaluating safety and efficacy of a telomerase peptide vaccination in patients with advanced hepatocellular carcinoma. BMC Cancer 2010; 10:209; PMID: 20478057
- Butterfield LH. Recent advances in immunotherapy for hepatocellular cancer. Swiss Med Wkly 2007; 137:83 - 90; PMID: 17370144
- Takayama T, Sekine T, Makuuchi M, Yamasaki S, Kosuge T, Yamamoto J, et al. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet 2000; 356:802 - 7; http://dx.doi.org/10.1016/S0140-6736(00)02654-4; PMID: 11022927
- Lee WC, Wang HC, Hung CF, Huang PF, Lia CR, Chen MF. Vaccination of advanced hepatocellular carcinoma patients with tumor lysate-pulsed dendritic cells: a clinical trial. J Immunother 2005; 28:496 - 504; http://dx.doi.org/10.1097/01.cji.0000171291.72039.e2; PMID: 16113606
- Kayashima H, Toshima T, Okano S, Taketomi A, Harada N, Yamashita Y, et al. Intratumoral neoadjuvant immunotherapy using IL-12 and dendritic cells is an effective strategy to control recurrence of murine hepatocellular carcinoma in immunosuppressed mice. J Immunol 2010; 185:698 - 708; http://dx.doi.org/10.4049/jimmunol.0900187; PMID: 20498356
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711 - 23; http://dx.doi.org/10.1056/NEJMoa1003466; PMID: 20525992
- Mizukoshi E, Nakamoto Y, Arai K, Yamashita T, Sakai A, Sakai Y, et al. Comparative analysis of various tumor-associated antigen-specific t-cell responses in patients with hepatocellular carcinoma. Hepatology 2011; 53:1206 - 16; http://dx.doi.org/10.1002/hep.24149; PMID: 21480325
- Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010; 28:3167 - 75; http://dx.doi.org/10.1200/JCO.2009.26.7609; PMID: 20516446
- Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med 2010; 207:2187 - 94; http://dx.doi.org/10.1084/jem.20100643; PMID: 20819927
- Mahnke K, Schonfeld K, Fondel S, Ring S, Karakhanova S, Wiedemeyer K, et al. Depletion of CD4+CD25+ human regulatory T cells in vivo: kinetics of Treg depletion and alterations in immune functions in vivo and in vitro. Int J Cancer 2007; 120:2723 - 33; http://dx.doi.org/10.1002/ijc.22617; PMID: 17315189
- Cheever MA. Twelve immunotherapy drugs that could cure cancers. Immunol Rev 2008; 222:357 - 68; http://dx.doi.org/10.1111/j.1600-065X.2008.00604.x; PMID: 18364014
- Pardee AD, McCurry D, Alber S, Hu P, Epstein AL, Storkus WJ. A therapeutic OX40 agonist dynamically alters dendritic, endothelial, and T cell subsets within the established tumor microenvironment. Cancer Res 2010; 70:9041 - 52; http://dx.doi.org/10.1158/0008-5472.CAN-10-1369; PMID: 21045144
- Zhu AX, Duda DG, Sahani DV, Jain RK. HCC and angiogenesis: possible targets and future directions. Nat Rev Clin Oncol 2011; 8:292 - 301; http://dx.doi.org/10.1038/nrclinonc.2011.30; PMID: 21386818
- Garbi N, Arnold B, Gordon S, Hammerling GJ, Ganss R. CpG motifs as proinflammatory factors render autochthonous tumors permissive for infiltration and destruction. J Immunol 2004; 172:5861 - 9; PMID: 15128765
- Niethammer AG, Xiang R, Becker JC, Wodrich H, Pertl U, Karsten G, et al. A DNA vaccine against VEGF receptor 2 prevents effective angiogenesis and inhibits tumor growth. Nat Med 2002; 8:1369 - 75; http://dx.doi.org/10.1038/nm1202-794; PMID: 12415261
- Bonder CS, Norman MU, Swain MG, Zbytnuik LD, Yamanouchi J, Santamaria P, et al. Rules of recruitment for Th1 and Th2 lymphocytes in inflamed liver: a role for alpha-4 integrin and vascular adhesion protein-1. Immunity 2005; 23:153 - 63; http://dx.doi.org/10.1016/j.immuni.2005.06.007; PMID: 16111634
- John B, Crispe IN. Passive and active mechanisms trap activated CD8+ T cells in the liver. J Immunol 2004; 172:5222 - 9; PMID: 15100260
- Hirano S, Iwashita Y, Sasaki A, Kai S, Ohta M, Kitano S. Increased mRNA expression of chemokines in hepatocellular carcinoma with tumor-infiltrating lymphocytes. J Gastroenterol Hepatol 2007; 22:690 - 6; PMID: 17444857
- Sato E, Fujimoto J, Toyoki H, Sakaguchi H, Alam SM, Jahan I, et al. Expression of IP-10 related to angiogenesis in uterine cervical cancers. Br J Cancer 2007; 96:1735 - 9; http://dx.doi.org/10.1038/sj.bjc.6603790; PMID: 17505511
- Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol 2008; 8:59 - 73; http://dx.doi.org/10.1038/nri2216; PMID: 18097448
- Nowak AK, Lake RA, Marzo AL, Scott B, Heath WR, Collins EJ, et al. Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor-specific CD8 T cells. J Immunol 2003; 170:4905 - 13; PMID: 12734333
- Ayaru L, Pereira SP, Alisa A, Pathan AA, Williams R, Davidson B, et al. Unmasking of alpha-fetoprotein-specific CD4(+) T cell responses in hepatocellular carcinoma patients undergoing embolization. J Immunol 2007; 178:1914 - 22; PMID: 17237442
- Zerbini A, Pilli M, Fagnoni F, Pelosi G, Pizzi MG, Schivazappa S, et al. Increased immunostimulatory activity conferred to antigen-presenting cells by exposure to antigen extract from hepatocellular carcinoma after radiofrequency thermal ablation. J Immunother 2008; 31:271 - 82; http://dx.doi.org/10.1097/CJI.0b013e318160ff1c; PMID: 18317360
- Mizukoshi E, Nakamoto Y, Arai K, Yamashita T, Mukaida N, Matsushima K, et al. Enhancement of tumor-specific T-cell responses by transcatheter arterial embolization with dendritic cell infusion for hepatocellular carcinoma. Int J Cancer 2010; 126:2164 - 74; PMID: 19739081
- Lok AS, Everhart JE, Wright EC, Di Bisceglie AM, Kim HY, Sterling RK, et al. Maintenance peginterferon therapy and other factors associated with hepatocellular carcinoma in patients with advanced hepatitis C. Gastroenterology 2011; 140:840 - 9; http://dx.doi.org/10.1053/j.gastro.2010.11.050; PMID: 21129375
- Astsaturov I, Petrella T, Bagriacik EU, de Benedette M, Uger R, Lumber G, et al. Amplification of virus-induced antimelanoma T-cell reactivity by high-dose interferon-alpha2b: implications for cancer vaccines. Clin Cancer Res 2003; 9:4347 - 55; PMID: 14555505
- Avella DM, Li G, Schell TD, Liu D, Shao-Min Zhang S, Lou X, et al. Regression of established hepatocellular carcinoma is induced by chemo-immunotherapy in an orthotopic murine model. Hepatology 2011; in press; http://dx.doi.org/10.1002/hep.24652; PMID: 21898502
- Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet 2002; 31:339 - 46; http://dx.doi.org/10.1038/ng0802-339; PMID: 12149612
- Iizuka N, Oka M, Yamada-Okabe H, Mori N, Tamesa T, Okada T, et al. Comparison of gene expression profiles between hepatitis B virus- and hepatitis C virus-infected hepatocellular carcinoma by oligonucleotide microarray data on the basis of a supervised learning method. Cancer Res 2002; 62:3939 - 44; PMID: 12124323
- Unitt E, Marshall A, Gelson W, Rushbrook SM, Davies S, Vowler SL, et al. Tumour lymphocytic infiltrate and recurrence of hepatocellular carcinoma following liver transplantation. J Hepatol 2006; 45:246 - 53; http://dx.doi.org/10.1016/j.jhep.2005.12.027; PMID: 16580084
- Schnitzbauer AA, Schlitt HJ, Geissler EK. Influence of immunosuppressive drugs on the recurrence of hepatocellular carcinoma after liver transplantation: a gap between basic science and clinical evidence. Transplantation 2011; 91:1173 - 6; http://dx.doi.org/10.1097/TP.0b013e318215e72b; PMID: 21427632