Abstract
Glycoproteins, as valuable targets for dendritic cell (DC)-vaccination in cancers, remain an open question. Glycosylated structures, which are aberrantly modified during cancerisation, impact positively or negatively on glycoprotein immunogenicity. Here is presented an oncofetal glycovariant of bile-salt-dependent-lipase, expressed on human tumoral pancreas and efficiently processed by DC’s, inducing T-lymphocyte activation.
This mini-review presents recent findings defining the interaction mechanisms between glycosylated tumor-associated antigen (TAA) and immune cells in order to develop dendritic cell (DC) vaccination in pancreatic ductal adenocarcinoma (PDAC).
DC’s initiate and regulate T-cell immunity and thus represents promising vectors for the clinical development of cancer vaccines. DC’s are endowed with two critical features: subsets and functional plasticity related to maturation state. These features direct them to mount functionally distinct types of responses. Besides, the quality of CD4+ T-cell responses is guided by the nature of the antigen. T cells, in addition to their peptide-specific reactions, can also respond to DC’s major histocompatibility complex (MHC) class I- and II-restricted peptides bearing motifs with post-translational modifications that include phophorylation and glycosylation (for a review see ref. Citation1). Aberrant glycosylations can dramatically lead the immune system to opposite issues. They can be related to autoimmune diseases where abnormally glycosylated proteins activate effector T cells responsible for autoimmune cytotoxic responses or to cancers,Citation2 where T-cell activation could be beneficial. Such an aberrant glycosylation of cell surface mucins distinguishes neoplastic from normal cells. Alteration in the glycan moiety, without any change in the polypeptide backbone, can then create neo-antigens on cells and may affect their interaction with the immune system. These onco-glycopeptides are therefore challenging antigens, and should encourage efforts on targeting of tumor-specific glycopeptides, without the risk of breaking tolerance to self. The notion that glycopeptides may be then recognized as foreign, while peptides, although immunogenic, are perceived as self is elegantly illustrated in a recent report on the glycopeptides neoepitopes derived from the most studied TAA, mucin MUC-1.Citation3
DC’s are equipped with specialized receptors, including Toll-like and lectin-like receptors (for a review see ref. Citation4). C-type lectin receptors recognize “specific” carbohydrate structures and participate in their detection during tumor progression, as shown in human PDAC.Citation5 Carbohydrate ligand receptors (CLRs), mannose receptor (MR), DC-SIGN, and dectin-1, to name but a few, are involved in glycan-mediated pathogen recognition and internalization of endogenous and exogenous antigen for loading on MHC class I and II molecules (for a review see ref. Citation6). Targeting antigen to MR enhances humoral and cellular responses and tumor-protective immune responses.Citation7 MR has also been demonstrated to play an enhancer role in antigen uptake and presentation since mannosylated antigens are better internalized and presented to T-cells.Citation8 However, depending on the DCs activation state, antigen presentation to T-cells might result in immune activation or immune downregulation. For instance, high-mannose structures on the cell wall target MR and DC-SIGN to induce signaling, thus leading to the production of anti-inflammatory cytokines such as IL-10.Citation6 Tumor-derived mucins, probably interacting with MR or other C-type lectin receptors on DC’s, were shown to affect the differentiation and maturation of monocyte DC’s. This then results in antigen presenting cells (APCs) with a tolerogenic/regulatory cytokine profile.Citation9 Yet, the in vivo situation is likely more complex; as shown by others, semi-maturated DC’s remain responsive to further signals in vitro and in vivo, which converts their tolerogenicity into immunogenicity.Citation10
Although the altered glycosylated tumor antigens represent potential targets for DC-based cancer immunotherapy, the outcome of glycosylated antigen transport, processing, and presentation to T-cells is indeed far less explored than that of peptidic antigens. Therefore, investigations of the interaction between tumor-specific glycosylated antigen and CLRs on APCs might explain why immune responses against tumor glycans are in general so difficult to obtain. They might also lead to the development of protocols that generate T-cells that are directed against glycan-containing peptides.Citation4
We have studied interactions between DC’s and the oncofetal fucose-rich glycovariant of bile salt-dependent lipase (BSDL), referred to as pathological BSDL carrying the fucosylated J28 glycotope (pBSDL-J28) because it is characterized by the J28 monoclonal antibody (mAbJ28).Citation11 pBSDL-J28 is expressed on human tumoral pancreasCitation12 and on human pancreatic tumor cell lines.Citation13 mAbJ28 recognizes a carbohydrate-dependent antigenic structure, termed J28 glycotope, located within the O-glycosylated mucin-like C-terminal domain of pBSDL-J28.Citation14 The formation of the J28 glycotope, characterized by fucosylated O-linked side chains, requires core2 β1,6 N-acetylglucosaminyltransferase and α1,3/4 fucosyltransferase,Citation14 two glycosyltransferases whose expression is upregulated during pancreatic neoplastic processes.Citation15
We previously reported the presence of circulating antibodies recognizing the O-glycosylated C-terminal domain in most type 1 diabetic patients and some patients afflicted with PDAC,Citation16 which may reflect the potential of this O-glycosylated C-terminal domain to induce humoral immunity. The expression of pBSDL-J28 on pancreatic tumoral cells and tissues and their immunogenic potential led us to hypothesize that this glycotope may serve as DC’s target and further lead to immunotherapy against PDAC. We therefore investigated the DC’s-processing of tumoral pancreatic glycoprotein pBSDL-J28 and its outcome.
shows that human immature monocyte-derived DC’s (iMoDC) captures pBSDL-J28 as well as recombinant C-terminal polypeptide-J28. pBSDL-J28 binds to MR (CD206) expressed on iMoDC surface.Citation12 Thus, oligosaccharides recognized by C-type lectin-like carbohydrate recognition domains of MR terminating in mannose, N-acetylglucosamine, and/or fucose residues, the latter being crucial elements of the J28 glycotope structure for mAbJ28 recognition, can be involved in DC’s binding (for a review see ref. Citation17). However, we cannot rule out that pBSDL-J28 binds to other receptors. Binding results in the internalization of pBSDL-J28 and its delivery into MHC class II compartment (MIIC) and late endosomes, where the Alexa 488-labeled pBSDL-J28 (A488-pBSDL-J28) processing products were detected. A488-(glycosylated)-peptide epitopes co-localized in lysosomes with LAMP-1 (CD107a) and in late endosomes with HLA-DM. Thus, DC’s internalize tumoral antigens mainly by receptor mediated-endocytosis but also by macropinocytosis (not shown here) to direct them to MIIC.Citation12
Figure 1. Uptake of J28 glycosylated antigen by iMoDC and intracellular localization. (A) Alexa 488-labeled pBSDL-J28 (A488-pBSDL-J28) and Alexa 488-labeled ovalbumin (A488-OVA) uptakes were analyzed by confocal laser microscopy. After 5-d culture, iMoDC were loaded with A488-pBSDL-J28 (50 μg/mL) or A488-OVA (50 μg/mL) for 1h, washed, fixed, and counterstained with CD1a antibodies followed by mouse Alexa-594-secondary antibodies. (B) Uptake of Alexa 488 recombinant C-terminal glycopolypeptide-carrying J28 glycotope (A488-Cter-J28) by iMoDC. iMoDC were incubated for 1h at 37°C with A488-Cter-J28, washed, counterstained with CD1a antibodies followed by mouse Alexa-594-secondary antibodies, and analyzed by confocal microscopy. (C) Intracellular localization of A488-pBSDL-J28. iMoDC were incubated for 1h at 37°C with A488-pBSDL-J28 (50 µg/mL) and counterstained with antibodies directed against CD206, HLA-DR, HLA-DM, and CD107a. Colocalization of each intracellular marker (red) with A488-pBSDL-J28 (green) is indicated in yellow. Original magnification x630.
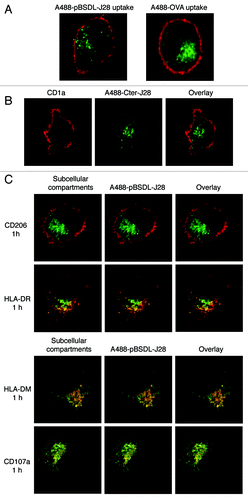
Hence pBSDL-J28 can be delivered into the HLA class II pathway, as opposed to MUC1 and HER2/Neu,Citation18 which present structures that are differently glycosylated. In PDAC, pBSDL-J28 carries fucosylated O-linked branches whereas tumoral MUC1 carries short O-glycans such as T (Galβ1–3GalNAcα1-O-Ser/Thr), Tn (GalNAcα1-O-Ser/Thr), and sialyl-Tn antigens (NeuAc-GalNAcα1-O-Ser/Thr).Citation5,Citation19 Several apparently contradictory reports have illustrated the complexity of glycoantigen binding and internalization. MUC1 binding and internalization involve the presence of sialylCitation19 and GalNAc residue at terminal position,Citation5 which require SIGLECs and MGL receptors respectively and can drastically change the binding affinity of ligand to receptors. These data confirm that the TAA glycosylation pattern plays an important role in antigen internalization, processing, glycosylated epitope presentation, and subsequent immune response.Citation3 Thus, in addition to regulating cell-cell adhesion and migration, the alteration of carbohydrates on the cell surface during carcinogenesis might influence the interaction with CLRs on resident APCs and direct the response toward T-lymphocyte activation or inhibition.Citation4
Licensing of DC’s by CD40 ligation remains the way to induce IL-12 production and subsequent Th1 polarized response, which is one of the objectives for cancer vaccination. This proved promising in our conditions as pulsing DC’s with pBSDL-J28 C-terminal glycopolypeptide and maturation with CD40L triggered CD4 and CD8 T-cell proliferation as shown in . Thus, DC’s could use distinct endocytosis mechanisms to simultaneously introduce pBSDL-J28 into separate intracellular compartments, which were dedicated to presentation to CD8+ or CD4+ T cells. Interestingly, it has been shown that the DC’s and macrophages use only MR-endocytosed OVA antigen for CD8+ T cell activation, whereas (macro)pinocytosed and scavenger receptor-endocytosed OVA antigen were used only for CD4+ T-cell activation.Citation20 Moreover, DC’s pulsed with either synthetic non-glycosylated pBSDL-C-terminal polypeptide or defucosylated pBSDL-J28 C-terminal glycopolypeptide induced only weak T-cell proliferation. This reduced T cell proliferation provides evidence for the pivotal implication of the fucose in the epitope recognition. Thus, these data highlight the existence of TCR(s) for pBSDL-J28 fucosylated and O-glycosylated C-terminal domain in the T-cell repertoire.
Figure 2. T-cell activation triggered by DC loaded with pBSDL-J28 C-terminal glycopolypeptide and exposed to CD40L (A) Proliferation of CD8+ and CD4+ T-lymphocytes. The histogram shows the proliferation of CD3+ T-lymphocytes (left panel: CD8 T-lymphocytes; right panel: CD4+ T-lymphocytes) cultured with DC’s matured with CD40L and incubated without antigen, (upper panel), with pBSDL-J28 C-terminal glycopolypeptide (Cter-J28) (middle/high panel), with defucosylated pBSDL-J28 C-terminal glycopolypeptide (Cter-Def) (middle/low panel), or with synthetic non-glycosylated pBSDL-C-terminal polypeptide (Cter-Synt) (lower panel). (B) Similar increased percent of proliferating CD4+ and CD8+ T-lymphocytes after culture with mature MoDC loaded with the pBSDL-J28 C-terminal glycopolypeptide, with defucosylated pBSDL-J28 C-terminal glycopolypeptide or with synthetic non-glycosylated pBSDL-C-terminal polypeptide.
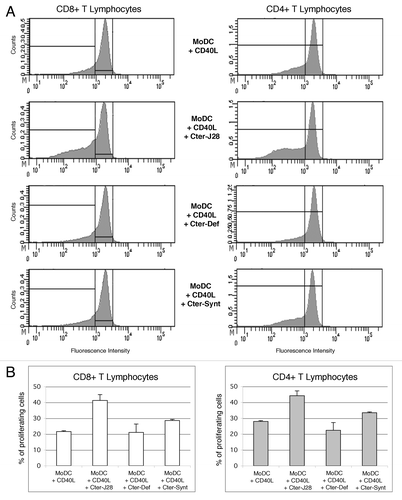
The demonstration that pBSDL-J28 is efficiently bound to DC MR, is also internalized by macropinocytosis and is further delivered to the MHC compartments to mount CD4+- and CD8+-T cell immunity makes it a potential mechanism for the targeting of DC’s and the delivery of tumor-associated epitopes for presentation. As long as TAA-specific immune responses recently highlighted in prostate and renal cancersCitation21 are considered as a key parameter of efficacy, a major task must be to improve DC vaccines. This improved vaccine would be to elicit antigen-specific CD4+ and CD8+ T cells with high frequencies and high-quality and to generate long-lived memory T cells, so as to be clinically active.
Have glycopeptides a future in vaccine therapies? Recent developments in the synthesis of complex carbohydrates and glycopeptides have made it possible to construct glycoconjugate vaccine candidates for evaluation in pre-clinical and clinical trials. The Danishefsky team has reported the synthesis Globo-H, Lewis y, Lewis x, Lewis b, KH-1, MUC-1 and the Tn, STn and TF-antigens. Several of the antigens have also been synthesized in a clustered configuration in an attempt to improve immunogenicity.Citation22 Thus far, it appears that carbohydrate-conjugate vaccines are well supported without any auto-immune reactions. This is underscored when they are combined with powerful additives such as saponin QS-21 and the immunomodulator cyclophosphamide.Citation23 There are parallels between vaccine-induced antibody responses and the clinical course after immunization. Yet it should be noted that high titers of the high affinity IgG antibodies were difficult to elicit in most patients. However, it is now accepted that glycopeptides can mediate classical MHC-mediated immune responses. Thus, cytotoxic T lymphocytes (CTL), which, as opposed to helper T-cells, are expected to react with tumor cells, present an additional opportunity for glycopeptide-based cancer vaccines.
Because conventional therapy is inefficient for PDAC, the fourth leading cause of cancer death in western countries, immunotherapeutic approaches have become tempting alternatives. Interestingly, numerous studies demonstrate that the clinical efficacy of different therapeutic modalities, including radiotherapy and chemotherapy, such as anthracyclins, Ab therapy such as Herceptin (anti-Her-2/neu mAb) and ipilimumab (anti-CTLA-4 antibody) and other drugs such as Sunitinib (which seems to inhibit STA3) and Imatinib (kinase inhibitor) is influenced by the immune system (for a review see refs. Citation24 and Citation25). Thus combinations of different treatments with DC vaccines might become efficient in a synergistic manner. Also, delivering tumor antigens directly to DC’s in vivo using antibodies, targeting specific DC surface receptors, constitutes a new approach to vaccination. The pioneer studies in mice demonstrate that potent antigen-specific helper CD4+ and IFNγ-CD8+ T-cell immunity are triggered in vivo by specific delivery of antigen to DC’s.Citation26 Such a utilization of immunostimulatory ligands to restore DC’s functions in tumors is currently applied to humans (for a review see ref. Citation28).Citation27
The fact that the majority of cell proteins are glycosylated, and that protein glycosylation is known to be dysregulated in cancer cells, should encourage more effort on the targeting of tumor-specific glycopeptides. In view of therapeutic advantages within combined treatment protocols, understanding the interaction mechanisms between glycosylated TAA and DC’s shows the way for further strategies to induce appropriate Th1 immune responses against onco-glycosylated antigen-bearing tumor cells.
References
- Engelhard VH, Altrich-Vanlith M, Ostankovitch M, Zarling AL. Post-translational modifications of naturally processed MHC-binding epitopes. Curr Opin Immunol 2006; 18:92 - 7; http://dx.doi.org/10.1016/j.coi.2005.11.015; PMID: 16343885
- Schietinger A, Philip M, Yoshida BA, Azadi P, Liu H, Meredith SC, et al. A mutant chaperone converts a wild-type protein into a tumor-specific antigen. Science 2006; 314:304 - 8; http://dx.doi.org/10.1126/science.1129200; PMID: 17038624
- Ryan SO, Turner MS, Gariépy J, Finn OJ. Tumor antigen epitopes interpreted by the immune system as self or abnormal-self differentially affect cancer vaccine responses. Cancer Res 2010; 70:5788 - 96; http://dx.doi.org/10.1158/0008-5472.CAN-09-4519; PMID: 20587526
- Aarnoudse CA, Garcia Vallejo JJ, Saeland E, van Kooyk Y. Recognition of tumor glycans by antigen-presenting cells. Curr Opin Immunol 2006; 18:105 - 11; http://dx.doi.org/10.1016/j.coi.2005.11.001; PMID: 16303292
- Napoletano C, Rughetti A, Agervig Tarp MP, Coleman J, Bennett EP, Picco G, et al. Tumor-associated Tn-MUC1 glycoform is internalized through the macrophage galactose-type C-type lectin and delivered to the HLA class I and II compartments in dendritic cells. Cancer Res 2007; 67:8358 - 67; http://dx.doi.org/10.1158/0008-5472.CAN-07-1035; PMID: 17804752
- van Kooyk Y, Rabinovich GA. Protein-glycan interactions in the control of innate and adaptive immune responses. Nat Immunol 2008; 9:593 - 601; http://dx.doi.org/10.1038/ni.f.203; PMID: 18490910
- He LZ, Crocker A, Lee J, Mendoza-Ramirez J, Wang XT, Vitale LA, et al. Antigenic targeting of the human mannose receptor induces tumor immunity. J Immunol 2007; 178:6259 - 67; PMID: 17475854
- Tan MC, Mommaas AM, Drijfhout JW, Jordens R, Onderwater JJ, Verwoerd D, et al. Mannose receptor-mediated uptake of antigens strongly enhances HLA class II-restricted antigen presentation by cultured dendritic cells. Eur J Immunol 1997; 27:2426 - 35; http://dx.doi.org/10.1002/eji.1830270942; PMID: 9341789
- Monti P, Leone BE, Zerbi A, Balzano G, Cainarca S, Sordi V, et al. Tumor-derived MUC1 mucins interact with differentiating monocytes and induce IL-10highIL-12low regulatory dendritic cell. J Immunol 2004; 172:7341 - 9; PMID: 15187110
- Voigtländer C, Rössner S, Cierpka E, Theiner G, Wiethe C, Menges M, et al. Dendritic cells matured with TNF can be further activated in vitro and after subcutaneous injection in vivo which converts their tolerogenicity into immunogenicity. J Immunother 2006; 29:407-15; PMID; 16799336; DOI: 10.1097/01.cji.0000210081.60178.b4.
- Mas E, Abouakil N, Roudani S, Miralles F, Guy-Crotte O, Figarella C, et al. Human fetoacinar pancreatic protein: an oncofetal glycoform of the normally secreted pancreatic bile-salt-dependent lipase. Biochem J 1993; 289:609 - 15; PMID: 8424803
- Franceschi C, Collignon A, Isnardon D, Benkoel L, Vérine A, et al. A novel tumor-associated pancreatic glycoprotein is internalized by human dendritic cells and induces their maturation. J Immunol 2011; 186:4067 - 77; http://dx.doi.org/10.4049/jimmunol.1000408; PMID: 21346236
- Panicot-Dubois L, Aubert M, Franceschi C, Mas E, Silvy F, Crotte C, et al. Monoclonal antibody 16D10 to the C-terminal domain of the feto-acinar pancreatic protein binds to membrane of human pancreatic tumoral SOJ-6 cells and inhibits the growth of tumor xenografts. Neoplasia 2004; 6:713 - 24; http://dx.doi.org/10.1593/neo.04298; PMID: 15720797
- Panicot L, Mas E, Pasqualini E, Zerfaoui M, Lombardo D, Sadoulet MO, et al. The formation of the oncofetal J28 glycotope involves core-2 beta6-N-acetylglucosaminyltransferase and alpha3/4-fucosyltransferase activities. Glycobiology 1999; 9:935 - 46; http://dx.doi.org/10.1093/glycob/9.9.935; PMID: 10460835
- Mas E, Pasqualini E, Caillol N, El Battari A, Crotte C, Lombardo D, et al. Fucosyltransferase activities in human pancreatic tissue: comparative study between cancer tissues and established tumoral cell lines. Glycobiology 1998; 8:605 - 13; http://dx.doi.org/10.1093/glycob/8.6.605; PMID: 9592127
- Panicot L, Mas E, Thivolet C, Lombardo D. Circulating antibodies against an exocrine pancreatic enzyme in type 1 diabetes. Diabetes 1999; 48:2316 - 23; http://dx.doi.org/10.2337/diabetes.48.12.2316; PMID: 10580419
- Taylor PR, Martinez-Pomares L, Stacey M, Lin HH, Brown GD, Gordon S. Macrophage receptors and immune recognition. Annu Rev Immunol 2005; 23:901 - 44; http://dx.doi.org/10.1146/annurev.immunol.23.021704.115816; PMID: 15771589
- Hiltbold EM, Vlad AM, Ciborowski P, Watkins SC, Finn OJ. The mechanism of unresponsiveness to circulating tumor antigen MUC1 is a block in intracellular sorting and processing by dendritic cells. J Immunol 2000; 165:3730 - 41; PMID: 11034378
- Carlos CA, Dong HF, Howard OM, Oppenheim JJ, Hanisch FG, Finn OJ. Human tumor antigen MUC1 is chemotactic for immature dendritic cells and elicits maturation but does not promote Th1 type immunity. J Immunol 2005; 175:1628 - 35; PMID: 16034102
- Burgdorf S, Kautz A, Böhnert V, Knolle PA, Kurts C. Distinct pathways of antigen uptake and intracellular routing in CD4 and CD8 T cell activation. Science 2007; 316:612 - 6; http://dx.doi.org/10.1126/science.1137971; PMID: 17463291
- Draube A, Klein-Gonźlez N, Mattheus S, Brillant C, Hellmich M, Engert A, et al. Dendritic cell based tumor vaccination in prostate and renal cell cancer: a systematic review and meta-analysis. PLoS ONE 2011; 6:e18801; http://dx.doi.org/10.1371/journal.pone.0018801; PMID: 21533099
- Zhu J, Warren JD, Danishefsky SJ. Synthetic carbohydrate-based anticancer vaccines: the Memorial Sloan-Kettering experience. Expert Rev Vaccines 2009; 8:1399 - 413; http://dx.doi.org/10.1586/erv.09.95; PMID: 19803761
- Buskas T, Thompson P, Boons GJ. Immunotherapy for cancer: synthetic carbohydrate-based vaccines. Chem Commun (Camb) 2009; 36:5335 - 49; http://dx.doi.org/10.1039/b908664c; PMID: 19724783
- Schuler G. Dendritic cells in cancer immunotherapy. Eur J Immunol 2010; 40:2123 - 30; http://dx.doi.org/10.1002/eji.201040630; PMID: 20853498
- Palucka K, Ueno H, Banchereau J. Recent developments in cancer vaccines. J Immunol 2011; 186:1325 - 31; http://dx.doi.org/10.4049/jimmunol.0902539; PMID: 21248270
- Bonifaz LC, Bonnyay DP, Charalambous A, Darguste DI, Fujii S, Soares H, et al. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J Exp Med 2004; 199:815 - 24; http://dx.doi.org/10.1084/jem.20032220; PMID: 15024047
- Klechevsky E, Flamar AL, Cao Y, Blanck JP, Liu M, O'Bar A, et al. Cross-priming CD8+ T cells by targeting antigens to human dendritic cells through DCIR. Blood 2010; 116:1685 - 97; http://dx.doi.org/10.1182/blood-2010-01-264960; PMID: 20530286
- Apetoh L, Locher C, Ghiringhelli F, Kroemer G, Zitvogel L. Harnessing dendritic cells in cancer. Semin Immunol 2011; 23:42 - 9; http://dx.doi.org/10.1016/j.smim.2011.01.003; PMID: 21295491