Abstract
Inflammation is a major stimulus for carcinogenesis; however inflammation can also inhibit tumor growth and deplete malignant cells. The differences between cancer-promoting and cancer-inhibitory inflammation are not clear. We identified Interferon-γ as a major mediator of cancer-inhibitory inflammation that promotes anti-cancer immunity in the liver and sensitizes malignant hepatocytes for apoptosis.
Inflammation is a critical promoter of carcinogenesis. Indeed, a characteristic of most, if not all tumors is a microenvironment of inflammatory cells and mediators that enables the growth of malignant cells and their acquisition of the ‘hallmarks of cancer’.Citation1 Moreover, chronic inflammation predisposes to several types of cancer, such as hepatocellular carcinoma.Citation2 However, inflammation has also the capacity to inhibit carcinogenesis by depleting malignant cells, and even to induce regression of established tumors.Citation2,Citation3 Currently, our knowledge of what makes the difference between tumor-promoting and tumor-inhibitory inflammation is rather limited. To develop more effective anti-tumor therapies, we need to define key inflammatory mediators and pathways that can turn tumor-promoting inflammation into anti-cancer immunity.
Hepatocellular carcinoma is a paradigm for inflammation-induced cancer, since it develops most frequently on grounds of chronic liver inflammation, consecutive tissue damage and compensatory regeneration.Citation2 An important component of the tumor-promoting inflammatory infiltrate are tumor-associated macrophages that exhibit an M2 phenotype and promote tissue remodeling and angiogenesis.Citation2 The initiation of liver cancer has been linked to activation of the NFκB pathway in liver macrophages and the secretion of interleukin-6, which, in turn, stimulates the STAT3 pathway in hepatocytes and enables the survival and proliferation of damaged cells.Citation4,Citation5 Inflammatory lymphocytes can also secrete STAT3-activating cytokines, such as interleukin-22, that may further promote liver cancer.Citation6
However, the common notion that chronic liver inflammation unequivocally promotes hepatocarcinogenesis may be an over-simplification. Indeed, clinical observations suggests that some chronic inflammatory liver diseases, such as viral hepatitis, progress more commonly to liver cancer than others; e.g., autoimmune hepatitis. Moreover, we noticed that transgenic mice, which overexpress interferon-γ (IFNγ) in the liver and manifest lifelong liver inflammation with persistent liver damage and regeneration, are not prone to spontaneous liver cancer.Citation7,Citation8 Therefore, we induced chemical hepatocarcinogenesis in these mice by application of diethylnitrosamine.Citation8 Quite surprisingly, chemical hepatocarcinogenesis was suppressed in the IFNγ-transgenic mice despite overt liver injury.Citation8 Indeed, IFNγ-transgenic mice developed fewer and less advanced lesions, suggesting that IFNγ may suppress both the initiation and the promotion of liver cancer. The tumor-suppressive effect of IFNγ seemed to be mediated in part by increasing tumor immune surveillance by lymphocytes, indicated by a strong infiltration of IFNγ-transgenic livers with CD8 T cells, NKT and NK cells.Citation8 In addition, IFNγ seemed to prevent carcinogenesis also by direct effects on damaged or malignant hepatocytes.Citation8 IFNγ induced a sustained and non-refractory activation of the STAT1 pathway in hepatocytes, but only transient STAT3 activation.Citation8 This IFNγ-induced activation of the STAT1 pathway was associated with an activation of the p53 tumor suppressor pathway.Citation8 Indeed, IFNγ induced accumulation of p53 and sensitized hepatocytes to apoptotic cell death in response to genotoxic stress.Citation8 These findings indicate that the carcinogenic potential of chronic inflammation is determined by type and composition of its mediators, most notably the proportion of IFNγ-secreting lymphocytes in chronic inflammatory tissue infiltrates.
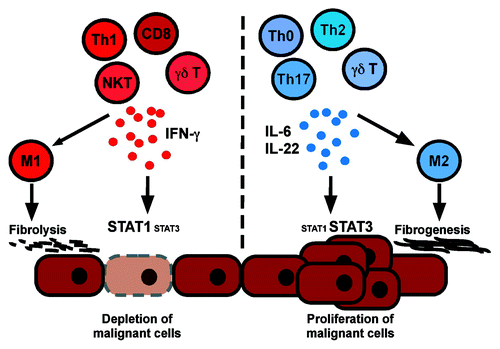
We thus propose that the differences between tumor-promoting and tumor-inhibitory liver inflammation may be explained not only by the degree of cytotoxic activity of inflammatory cells, but also by predominance of either STAT1-activating cytokines, such as IFNγ, or STAT3-activating cytokines, such as interleukin-6 or interleukin-22 (). This model is compatible with the finding that the balance of STAT1 and STAT3 activation seems to predict the clinical outcome in cutaneous melanoma.Citation9 During tumor promotion, the presence or absence of IFNγ further influences the tumor microenvironment and tissue remodelling. Indeed, IFNγ is known to promote macrophage polarization toward an M1 phenotype and to inhibit fibrogenesis, whereas M2 macrophages, which are induced by non-IFNγ producing lymphocytes and malignant hepatocytes, promote fibrogenesis and angiogenesis. It is conceivable that the presence of IFNγ during tumor promotion may prevent induction of M2 macrophages. In summary, inflammatory infiltrates with high content of IFNγ-secreting lymphocytes, i.e., Th1 cells, CD8 T cells, some NK cells and some NKT cells, may characterize tumor-suppressive inflammation, whereas infiltrates with high content of IFNγ non-producing or interleukin-22 producing lymphocytes may characterize tumor-promoting infiltrates. Therefore, manipulating the type of chronic inflammation and the composition of tumor-associated inflammatory infiltrates may serve the prevention of cancer.
Figure 1. A model of cancer-promoting and cancer-inhibitory liver inflammation. Tumor-suppressive inflammatory liver infiltrates are characterized by high content of IFNγ-secreting lymphocytes (Th1, CD8 T, NK and NKT cells), sustained activation of the STAT1 pathway in hepatocytes, macrophage polarization toward an M1 phenotype and fibrolysis. In contrast, tumor-promoting liver infiltrates are characterized by high content of IFNγ non-producing or interleukin-22 producing lymphocytes, interleukin-6 secretion by various cell types, macrophages with M2 phenotype, sustained STAT3 activation in hepatocytes, fibrogenesis and angiogenesis.
| Abbreviations: | ||
| IFNγ | = | interferon-γ |
References
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646 - 74; http://dx.doi.org/10.1016/j.cell.2011.02.013; PMID: 21376230
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature 2008; 454:436 - 44; http://dx.doi.org/10.1038/nature07205; PMID: 18650914
- Zitvogel L, Apetoh L, Ghiringhelli F, André F, Tesniere A, Kroemer G. The anticancer immune response: indispensable for therapeutic success?. J Clin Invest 2008; 118:1991 - 2001; http://dx.doi.org/10.1172/JCI35180; PMID: 18523649
- Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer 2009; 9:798 - 809; http://dx.doi.org/10.1038/nrc2734; PMID: 19851315
- He G, Karin MNF. -κB and STAT3 - key players in liver inflammation and cancer. Cell Res 2011; 21:159 - 68; http://dx.doi.org/10.1038/cr.2010.183; PMID: 21187858
- Park O, Wang H, Weng H, Feigenbaum L, Li H, Yin S, et al. In vivo consequences of liver-specific interleukin-22 expression in mice: Implications for human liver disease progression. Hepatology 2011; 54:252 - 61; http://dx.doi.org/10.1002/hep.24339; PMID: 21465510
- Toyonaga T, Hino O, Sugai S, Wakasugi S, Abe K, Shichiri M, et al. Chronic active hepatitis in transgenic mice expressing interferon-γ in the liver. Proc Natl Acad Sci USA 1994; 91:614 - 8; http://dx.doi.org/10.1073/pnas.91.2.614; PMID: 8290572
- Lüth S, Schrader J, Zander S, Carambia A, Buchkremer J, Huber S, et al. Chronic inflammatory IFN-γ signaling suppresses hepatocarcinogenesis in mice by sensitizing hepatocytes for apoptosis. Cancer Res 2011; 71:3763 - 71; http://dx.doi.org/10.1158/0008-5472.CAN-10-3232; PMID: 21512142
- Wang W, Edington HD, Rao UN, Jukic DM, Land SR, Ferrone S, et al. Modulation of signal transducers and activators of transcription 1 and 3 signaling in melanoma by high-dose IFNalpha2b. Clin Cancer Res 2007; 13:1523 - 31; http://dx.doi.org/10.1158/1078-0432.CCR-06-1387; PMID: 17332298