Abstract
Interleukin-33, a ligand for ST2/T1, has an important role in allergy, autoimmunity and inflammation. The role of IL-33/ST2 axis in cancer is not elucidated. Using metastatic breast cancer model we provide evidence that lack of ST2 signaling led to reduced tumor growth and metastasis and enhanced anti-tumor immunity.
Interleukin-33 (IL-33) is a member of the IL-1 family of cytokines and was identified as a natural ligand for ST2/T1, an orphan receptor in the IL-1R family. IL-33 is produced as a biologically active full-length molecule and may function as an alarmin, when released after cell damage or as nuclear IL-33 that reduces NF-kB–triggered proinflammatory genes expression in non–IL-33 receptor–mediated manner. Outside the cell, IL-33 acts as a classical cytokine by binding to its receptor, membrane bound ST2L (now designated IL-33Rα-chain) and IL-1R accessory protein (IL-1RAp), leading to NFkB and MAPK activation.Citation1,Citation2 These findings highlight the complex role of this multifunctional cytokine.
IL-33 is constitutively expressed in endothelial and epithelial cells of mucous membranes, keratinocytes and fibroblasts. ST2 (IL-33 receptor) exists as a full-length membrane molecule (ST2L) and as soluble, decoy variant ST2 (sST2). ST2L is expressed by T cells (Th2, but not Th1 cells), NK and NKT cells, mast cells, monocytes, dendritic cells and granulocytes. ST2L was shown to be stably and selectively expressed by murine Th2,Citation3 and also human Th2 cells and NKT-like cells.Citation4 IL-33 polarizes naïve T cells to produce IL-4, IL-5 and IL-13 (Th2-associated cytokines), potently induces pro-inflammatory cytokines and chemokines by mast cells and eosinophils and amplifies polarization of alternatively activated M2 macrophages. Although ST2 (and IL-33) may act primarily through a Th2-pathway, IL-33/ST2 axis can also promote Th1-type responses depending on the local conditions, for example, the presence or absence of IL-12.Citation1
IL-33 participates in many diseases with dual, proinflammatory or protective roles depending on the cellular and cytokine context.Citation1 Namely, IL-33 has a protective role during progression of atherosclerosis, obesity, TNF-α mediated bone loss and experimental fulminant hepatitis. We have demonstrated that ST2 deficiency led to more severe Con-A induced hepatitis associated with increased numbers of TNF-α, IFN-γ and IL-17 producing liver infiltrating mononuclear cells and increased systemic pro-inflammatory cytokines. Moreover, pre-treatment with IL-33 prior to Con-A injection led to attenuation of liver injury and increased liver CD4+Foxp3+ and IL-4 producing CD4+ T cells.Citation5
IL-33 is believed to be mainly involved in allergen-specific Th2-type inflammation and administration of neutralizing antibodies against ST2 or IL-33 was shown to attenuate eosinophilic pulmonary inflammation in the murine model of allergic asthma.Citation6 We have shown that deletion of ST2 enhanced disease induction or severity in several experimental models of organ specific autoimmunity. Thus, ST2−/− mice were more sensitive to induction of multiple low dose streptozotocin diabetes,Citation7 experimental allergic encephalomyelitis (EAE) (unpublished data) and Con-A induced fulminant hepatitis,Citation5 the findings that suggest anti-inflammatory effects of IL-33/ST2 axis. Indeed, Anthony et al.Citation8 have elegantly shown that intravenous immunoglobulins (ivIgs) suppress inflammation through novel Th2 pathway that involves IL-33. IgG crystallizable fragments stimulate the induction of IL-33 by dendritic cells and macrophages which promote IL-4 producing basophils that increase the expression of the inhibitory Fc receptor FcγRIIB on effector macrophages.
The data on the role of IL-33/ST2 axis in cancer are lacking. We provided the evidence that deletion of ST2 signaling may enhance anti-tumor immune response in a murine model of metastatic 4T1 breast carcinoma.Citation9 We showed delayed appearance of palpable primary tumor, slower tumor growth and reduced number and size of metastatic colonies in lungs and livers in ST2−/− mice. ST2 deletion led to increased absolute numbers of CD4+ and CD8+ T cells in local lymph nodes and spleens after tumor challenge. ST2−/− splenocytes, NK and CD8+ T cells had enhanced cytotoxicity with higher frequency of activated, NKp46+ CD107a+ cells NK cells both constitutively and after tumor inoculation. ST2−/− mice had increased numbers of IFN-γ expressing NK cells, while undetectable IL-10 producing NK cells. In addition, ST2 deficient mice had constitutively higher percentages of activated CD27highCD11bhigh NK cells, CD69+ and KLRG- NK cells. In vivo depletion of CD8+ or NK cells revealed the key role for NK cells in enhanced anti-tumor immunity in ST2−/− mice. ST2−/− mice had increased serum levels of IL-17, IFN-γ and TNF-α and decreased serum IL-4 levels after tumor challenge. Further, the lack of ST2 was associated with constitutively decreased frequencies of F4/80+CD206+ alternatively activated M2 macrophages in spleen (unpublished data), that may potently suppresses innate and adaptive anti-tumor immunity. The increased anti-tumor immune response observed in ST2-deficient mice seems to be based mainly on two independent mechanisms: Th1/Th17 cell polarization and enhanced NK cell cytotoxic activity.
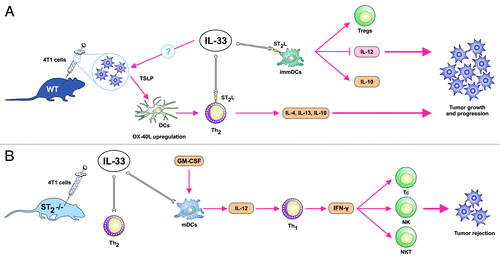
Based on our findings it could be speculated that in the absence of ST2 immunosuppressive Th2-type immune response cannot be enhanced in the presence of IL-33 from epithelial, endothelial or possibly tumor cells. In this setting, IL-12 produced by classically activated M1 macrophages promote maturation of DCs and consequently strong Th1/Th17 response that activate tumoricidal NK, NKT cells and CD8+ T cytotoxic lymphocytes (). Hypothetically, if IL-33 is overexpressed either endogenously or exogenously, its binding to ST2L activates Th2 cells and promotes generation of relatively immature dendritic cells that do not produce IL-12p70 (). Immature dendritic cells promote generation of T regs and therefore facilitate tumor progression and metastasis. In addition, ST2 signaling could possibly induce the production of thymic stromal lymphopoietin (TSLP) by tumor cells. TSLP is known to upregulate OX40L on dendritic cells and induce IL-4 and more importantly immunosuppressive IL-10 and IL-13 producing Th2-cells that promote cancer escape.Citation10
Figure 1. Hypothetical role of IL-33/ST2 axis in tumor growth and progression based on mouse mammary adenocarcinoma 4T1 cancer model. (A) The effects of endogenous and also exogenous IL-33 in tumor-bearing hosts. IL-33 via its receptor ST2L activates Th2-polarized cells and generates relatively immature dendritic cells that do not produce IL-12p70. Subsequently, ST2 signaling could possibly enhance production of thymic stromal lymphopoietin (TSLP) by tumor cells which by upregulating OX40L on dendritic cells lead to induction of IL-4, and more importantly immunosuppressive IL-10 and IL-13 producing Th2-cells that promote cancer escape. Immature dendritic cells induce T regs that contribute to an immunosuppressive environment and facilitate metastasis. (B) In the absence of ST2 (IL-33 receptor), IL-33 produced by epithelial cells and possibly tumor cells does not lead to the activation of Th2-associated immunosuppressive response. Concomitantly, IL-12 produced by classically activated M1 macrophages lead to the maturation of DC, strong IFN-γ production by Th1 cells which activate tumoricidal NK, NKT cells and CD8+ T cytotoxic lymphocytes.
Our preliminary data show that exogenously administrated IL-33 enhanced primary 4T1 tumor growth and inhibited innate anti-tumor immunity. Several questions remains to be answered: whether IL-33 facilitates cancer metastases, how IL-33 affects proliferation, differentiation and apoptosis of tumor cells, angiogenesis and phenotype and function of tumor-infiltrating immune cells.
References
- Liew FY, Pitman NI, McInnes IB. Disease-associated function of IL-33: the new kid in the IL-1 family. Nat Rev Immunol 2010; 10:103 - 10; http://dx.doi.org/10.1038/nri2692; PMID: 20081870
- Ali S, Mohs A, Thomas M, Klare J, Ross R. The Dual Function Cytokine IL-33 Interacts with the Transcription Factor NF-kB To Dampen NF-kB–Stimulated Gene Transcription. J Immunol 2011; 187:1609 - 16; http://dx.doi.org/10.4049/jimmunol.1003080; PMID: 21734074
- Xu D, Chan WL, Leung BP, Huang F, Wheeler R, Piedrafita D, et al. Selective expression of a stable cell surface molecule on type 2 but not type 1 helper T cells. J Exp Med 1998; 187:787 - 94; http://dx.doi.org/10.1084/jem.187.5.787; PMID: 9480988
- Chan WL, Pejnovic N, Lee CA, Al-Ali NA. Type 2 lymphocytes predominate in HIV patients: Direct analysis of subset specific surface markers ST2L and IL-18R. J Immunol 2001; 167:1238 - 44; PMID: 11466339
- Volarevic V, Mitrovic M, Milovanovic M, Zelen I, Nikolic I, Mitrovic S, et al. Protective role of IL-33/ST2 axis in Con A-induced hepatitis. J Hepatol 2011; http://dx.doi.org/10.1016/j.jhep.2011.03.022; PMID: 21703183
- Liu X, Li M, Wu Y, Zhou Y, Zeng L, Huang T. Anti-IL-33 antibody treatment inhibits airway inflammation in a murine model of allergic asthma. Biochem Biophys Res Commun 2009; 386:181 - 5; http://dx.doi.org/10.1016/j.bbrc.2009.06.008; PMID: 19508862
- Zdravkovic N, Shahin A, Arsenijevic N, Lukic ML, Mensah-Brown EP. Regulatory T cells and ST2 signaling control diabetes induction with multiple low doses of streptozotocin. Mol Immunol 2009; 47:28 - 36; http://dx.doi.org/10.1016/j.molimm.2008.12.023; PMID: 19356801
- Anthony RM, Kobayashi T, Wermeling F, Ravetch JV. Intravenous gammaglobulin suppresses inflammation through a novel TH2 pathway. Nature 2011; 475:110 - 3; http://dx.doi.org/10.1038/nature10134; PMID: 21685887
- Jovanovic I, Radosavljevic G, Mitrovic M, Juranic VL, McKenzie AN, Arsenijevic N, et al. ST2 deletion enhances innate and acquired immunity to murine mammary carcinoma. Eur J Immunol 2011; 41:1902 - 12; http://dx.doi.org/10.1002/eji.201141417; PMID: 21484786
- Olkhanud PB, Rochman Y, Bodogai M, Malchinkhuu E, Wejksza K, Xu M, et al. Thymic Stromal Lymphopoietin Is a Key Mediator of Breast Cancer Progression. J Immunol 2011; 186:5656 - 62; http://dx.doi.org/10.4049/jimmunol.1100463; PMID: 21490155