Abstract
Improving on the limited success of cancer immunotherapy requires new approaches to inhibit immunosuppressive pathways initiated by tumor cells to “escape” protective immunity. One unique approach utilizes Salmonella for systemic delivery of inhibitory RNA, targeting the immunosuppressive molecule Stat3, and a Survivin vaccine to suppress growth of aggressive murine tumors.
It is now accepted that tumors avoid destruction by the host immune system through a multitude of immunosuppressive pathways. Tumors are simultaneously capable of disabling protective T cells, activating Tregs and MDSCs, while secreting immunosuppressive molecules to further incapacitate anti-tumor immunity.Citation1 One of the very few approaches targeting suppressive mediators that has shown clinical efficacy is the FDA approved humanized antibody known as Ipilimumab or Yervoy™, which blocks CTLA-4 function and profoundly improves survival of some melanoma patients.Citation2 However, there was disappointment when Ipilimumab was combined with a melanoma-specific peptide vaccine and showed no improvement in survival. In addition, possible side effects by Ipilimumab can be life threatening (FDA box warning) which may limit its applicability to only patients with advanced disease. Nevertheless, the concept is attractive if the design of the combined immunotherapies could be improved.
In our study published in Cancer Research,Citation3 we utilized the unique properties of Salmonella as a tumor-homing vector and as a vaccine. This gave us the flexibility to target immunosuppressive molecules in the tumor using shRNA plasmid technology (shStat3-ST) and to utilize a strong Salmonella promoter to express tumor antigen for CTL induction (Max-ST) (). We targeted the multi-functional molecule Stat3, which has been repeatedly implicated in tumor survival, proliferation, angiogenesis and metastasis while promoting expression of immunosuppressive factors, Treg expansion and inhibition of TH1 immunostimulatory molecules.Citation4 Our logic in trying to inactivate Stat3 function was supported by promising results of small-molecule and siRNA inhibitors used in tumors with hyperactivated Stat3 phenotypes.Citation5 We combined inactivation of Stat3 with vaccination using Salmonella expressing a versatile TAA known as Survivin (SVN). SVN is a member of the inhibitor of apoptosis (IAP) protein family and possesses ideal TAA properties: undetectable expression in non-cancerous adult tissues, overexpression in most human tumors, and induces cytotoxic T lymphocytes.Citation6 SVN is regulated by many pathways including Stat3 transactivation through IL-11 signaling.Citation7
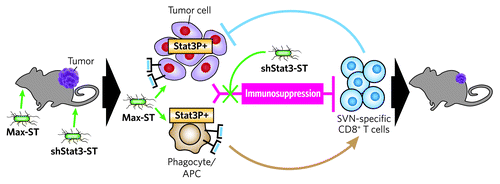
Figure 1. shStat3-ST treatment enhances Max-ST vaccination efficacy. Proposed mechanism of action by combined treatment with shStat3-ST and Max-ST. Left to right: In a B16F10 tumor-bearing mouse, shStat3-ST is first injected intravenously, followed by oral vaccination with Max-ST. Immunosuppression (red) developing from hyperactivated Stat3 expression (Stat3P+) in tumor cells and APCs is targeted by shStat3-ST (green line). Max-ST serves to boost anti-tumor responses through enhanced expression of SVN within tumor cells or APCs (green arrows) resulting in SVN antigen presentation via MHC class I. The combined treatment works synergistically to allow for tumor killing by SVN-specific CD8+ T cells (blue line) leading to tumor regression.
How did they accomplish the feat together, when both treatments had far less activity as single agents? Increased immunosuppression caused by larger tumors is possibly what rendered Max-ST less effective. The ineffectiveness of shStat3-ST alone could be explained by differences in its mode of action compared with other published Stat3 silencing strategies, for example CpG-Stat3 siRNA.Citation5 CpG-Stat3 siRNA has been shown to silence Stat3 in multiple subsets including CD11b+ myeloid cells, CD11c+ dendritic cells and CD19+ B cells in TDLNs, which may only occur because it is delivered peritumorally. shStat3-ST was shown to effectively silence Stat3 in F4/80+ macrophage subsets and it did so when delivered systemically. Furthermore, silencing by shStat3-ST in F4/80+ macrophages was not significantly different from that seen for CpG-Stat3 siRNA. Although peritumoral treatment with CpG-Stat3 siRNA showed some control against subcutaneous B16F10 growth, likely through modulation of Stat3 expression in multiple immune subsets, it is uncertain whether it would show any efficacy if delivered systemically.
Based on our study, we can theorize how the combined treatment worked synergistically to control larger tumors. Since the cellular target of shStat3-ST is macrophages, it is possible that the APC of choice for Max-ST could also be macrophages, either TAMs or those found in Peyer's patches. Thus, by silencing Stat3 in SVN-presenting macrophages, interacting T cells are more likely to become activated and proliferate, which could explain the increases in lymphocyte Ki67 levels we observed in tumor-bearing mice receiving the combined therapy, but not in groups receiving single treatments.Citation3 Alternatively, the silencing of Stat3 in more developed tumors may re-sensitize them to killing by SVN-specific responses generated outside the proximity of shStat3-ST effect. Techniques that utilize GFP or luciferase markers to track Salmonella in vivo could be used to study in depth whether shStat3-ST, given intravenously, and Max-ST, given orally, co-localize and infect the same cell targets or exert their effects in different cell subsets that eventually converge in TDLNs or in the tumor itself.
The treatment strategy of shStat3-ST followed by Max-ST is uniquely synergistic to interrupt a very specific immunosuppressive pathway in TAMs, since we could not duplicate therapeutic outcomes using Max-ST vaccination with shRNAs targeting other macrophage-derived suppressive molecules such as iNOS and arginase. Exploring possible changes to downstream events occurring in TAMs following shStat3-ST administration may give further insight into the intricate workings of the combined treatment. For example, the Stat3-dependent molecule IL-23, produced by TAMs, has been shown to activate Stat3 in Tregs, leading to increased production of the immunosuppressive molecule IL-10.
The combined strategy has already shown considerable efficacy in several tumor models. Because the timeframe for Stat3 silencing is finite, tumor escape eventually occurs, possibly aided by other survival and immunosuppression pathways that have yet to be defined. Therefore, silencing multiple immunosuppressive targets such as TGFβ, CTLA-4 and indoleamine 2,3-dioxygenase could result in better tumor control. The Salmonella technology could accommodate testing these new targets in combination with SVN vaccination. The excitement of this approach is the unique combination of RNA silencing and a vaccination strategy that have not previously failed clinically and a solid basis from molecular and organismic studies affirming its success. Similar to Ipilimumab, our Salmonella approach is systemic, giving it favorable translational feasibility over peritumoral approaches. Future clinical studies may confirm its mild toxicity in mice, suggesting a possible advantage over Ipilimumab in regards to patient safety from severe life-threatening side effects.
| Abbreviations: | ||
| APC | = | antigen-presenting cell |
| CTL | = | cytotoxic T lymphocyte |
| CTLA-4 | = | cytotoxic T-lymphocyte antigen 4 |
| MDSC | = | myeloid-derived suppressor cell |
| shRNA | = | short hairpin RNA |
| siRNA | = | small interfering RNA |
| Stat3 | = | signal transducer and activator of transcription 3 |
| TAA | = | tumor-associated antigen |
| TAM | = | tumor-associated macrophage |
| TDLN | = | tumor-draining lymph node |
| TGFβ | = | transforming growth factor β |
| Treg | = | regulatory T cell |
References
- Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol 2007; 25:267 - 96; http://dx.doi.org/10.1146/annurev.immunol.25.022106.141609; PMID: 17134371
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711 - 23; http://dx.doi.org/10.1056/NEJMoa1003466; PMID: 20525992
- Manuel ER, Blache CA, Paquette R, Kaltcheva TI, Ishizaki H, Ellenhorn JD, et al. Enhancement of cancer vaccine therapy by systemic delivery of a tumor-targeting Salmonella-based STAT3 shRNA suppresses the growth of established melanoma tumors. Cancer Res 2011; 71:4183 - 91; http://dx.doi.org/10.1158/0008-5472.CAN-10-4676; PMID: 21527558
- Kortylewski M, Yu H. Role of Stat3 in suppressing anti-tumor immunity. Curr Opin Immunol 2008; 20:228 - 33; http://dx.doi.org/10.1016/j.coi.2008.03.010; PMID: 18479894
- Kortylewski M, Swiderski P, Herrmann A, Wang L, Kowolik C, Kujawski M, et al. In vivo delivery of siRNA to immune cells by conjugation to a TLR9 agonist enhances antitumor immune responses. Nat Biotechnol 2009; 27:925 - 32; http://dx.doi.org/10.1038/nbt.1564; PMID: 19749770
- Schmidt SM, Schag K, Muller MR, Weck MM, Appel S, Kanz L, et al. Survivin is a shared tumor-associated antigen expressed in a broad variety of malignancies and recognized by specific cytotoxic T cells. Blood 2003; 102:571 - 6; http://dx.doi.org/10.1182/blood-2002-08-2554; PMID: 12576330
- Altieri DC. Validating survivin as a cancer therapeutic target. Nat Rev Cancer 2003; 3:46 - 54; http://dx.doi.org/10.1038/nrc968; PMID: 12509766