Abstract
Two recently published articles from Siegers and colleagues detail both a novel human gamma delta T cell (GDTc) expansion protocol and a bioluminescent xenograft model of Ph+ leukemia, in which GDTc adoptive therapy was tested. Additionally, B-cell chronic lymphocytic leukemia-derived cells were newly identified as targets of preferentially expanded Vdelta1 GDTc.
Anti-Leukemia Activity of Human Gamma Delta T Cells
Once an obscure cell type receiving little attention, gamma delta T cells (GDTc) are now coming into their own, as new studies are published at an ever-increasing rate. GDTc are immunosurveillance cells, comprising 2–5% of circulating lymphocytes in humans. Ensslin initially reported on MHC-unrestricted GDTc lysis of tumor cell lines.Citation1 We have since learned that GDTc recognition and tumor lysis can be mediated by T cell antigen receptor (TCR), the natural killer receptor NKG2D, as well as fas-fasL interactions, depending on GDTc subset and target.
Promising reports from recent clinical trials support the use of GDTc as immunotherapeutic agents; either via adoptive transferCitation2 or in vivo stimulation with compounds such as IPH1101Citation3 or the aminobisphosphonate zoledronate.Citation4 While the autologous setting is preferable for adoptive transfer, a report of allotransplantation without the incidence of graft-vs.-host disease suggests that this is also clinically feasible.Citation5
The study of GDTc has recently been rendered more accessible with the advent of magnetic cell separation allowing positive or negative selection of GDTc from blood or tissues. With the ultimate goal of clinical translation, we used this technology to develop a novel protocol to isolate human GDTc from peripheral blood and expand pure populations to clinically relevant numbers, without the use of irradiated feeder cells. Additionally, we identified new GDTc subset-specific targets and presented results from preclinical immunotherapy experiments.Citation6,Citation7
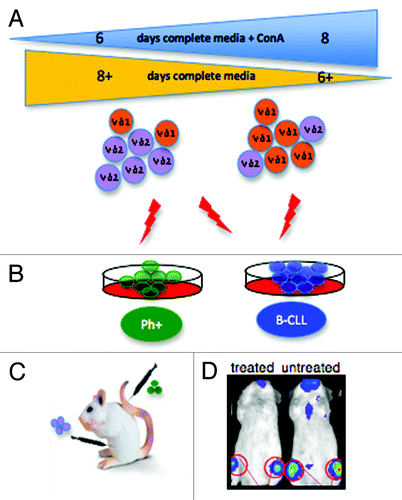
Our GDTc expansion protocol expands both Vgamma9Vdelta2 GDTc (Vδ2) and also the lesser-prevalent Vdelta1 GDTc (Vδ1) subset from human blood.Citation6 A correlation between duration of initial GDTc exposure to Concanavalin A (ConA) and expansion of Vδ1 was evident (). Bartkowiak and colleagues reported an expanded population of Vδ1 in the blood of B-CLL patients.Citation8 Thus, using mixed Vδ1/Vδ2 cultures, the CD107 mobilization assayCitation9 was employed to simultaneously test activation of GDTc subsets against Philadelphia chromosome positive (Ph+) or B-cell chronic lymphocytic leukemia (B-CLL)-derived cells. Indeed, this approach could be applied to any target of interest. While Ph+ EM2 cells only activated Vδ2, both Vδ1 and Vδ2 were activated by B-CLL-derived MEC1 and TMD2 (), thus obviating the need to separate subsets for possible future autologous cellular therapy of B-CLL. An interesting line of study made uniquely possible by generation of mixed cultures is the influence exerted by one GDTc subset on the other; for example, one could explore whether Vδ2 activation suppresses Vδ1.
Figure 1. (A) Gamma delta T cells (GDTc) isolated from human blood were cultured for six to eight days in complete medium containing Concanavalin A (ConA). Vdelta1 (Vδ1) or Vdelta2 (Vδ2) subset prevalence in expanded culture correlated with the duration of exposure to ConA. (B) Vδ2 GDTc are cytotoxic to EM-2eGFPluc cells, Ph+ leukemia cells transduced with a lentiviral vector encoding eGFP and luciferase. Both Vδ1 and Vδ2 are cytotoxic to B-CLL-derived MEC1 and TMD2 cell lines. (C) The bioluminescent xenograft Ph+ leukemia model was established via intravenous (IV) injection of EM-2eGFPluc cells. Vδ2 GDTc therapy was investigated; infusion of GDTc intraperitoneally gave rise to better engraftment than when GDTc were injected IV. (D) Leukemia progression was monitored in vivo via IVIS® technology (In Vivo Imaging System, Xenogen). Shown here are representative examples of treated and leukemia-bearing mice. Red circles are regions of interest from which bioluminescence values were quantified.
Finally, some indications as to which receptors play a role in GDTc cytotoxicity against their targets were obtained by correlating surface marker expression with differential cytotoxicity exhibited by a panel of Vδ1 and Vδ2 clones. TCR, CD56 and CD95 expression correlated with Vδ1 cytotoxicity, while both TCR and NKG2D were implicated in that of Vδ2. Although perhaps unconventional, using this panel of clones proved advantageous over the antibody blocking experiments typically employed to elucidate mechanism. Initial attempts at blocking failed when FcR-mediated apoptosis occurred in B-CLL-derived cells, incited even by isotype control antibodies. Further verification of results via blocking will require the generation of Fab fragments.
Comparing antigen expression on target cell lines in parallel provided additional information. The absence of HLA class I on the surface of EM2eGFPluc cells pointed to the “missing self” mechanism by which Daudi lymphoma cells are killed by Vδ2.Citation10 TRAILR1 expression was elevated on B-CLL derived cell lines but not EM2eGFPluc, suggesting involvement of this surface antigen in Vδ1 cytotoxicity. Much work lies ahead to further elucidate these cytotoxic mechanisms.
Our other study focused on expanding Vδ2 GDTc for use in a novel bioluminescent xenograft model of Ph+ leukemia.Citation7 EM-2 cells expressing eGFP and luciferase were injected intravenously (IV) into NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ mice (), closely emulating human pathogenesis, with leukemia engraftment restricted largely to the bone marrow, as monitored in live mice via IVIS® technology (, In Vivo Imaging System, Xenogen). Expanded GDTc were cytotoxic to Ph+ leukemia cell lines in vitro and thus administered in vivo with the goal of eradicating leukemia. Intraperitoneal injection elicited enhanced GDTc engraftment compared with IV. Although different dosages were attempted, no apparent survival advantage was achieved. However, reduced leukemia burden was evidenced in some treated mice. In the absence of an intact immune system, tumor burden quickly progressed to a blast crisis state, overwhelming GDTc. Potential improvements for future studies include: lowering the initial leukemia dose; increasing the number and frequency of GDTc administration; raising IL-2 doses; or injecting GDTc in the context of whole peripheral blood mononuclear cells.
We have reported on a new protocol that can be manipulated to preferentially expand Vδ1 or Vδ2 subsets from human blood. Vδ1 are cytotoxic to B-CLL-derived cells, suggesting that Vδ1 may represent a novel cellular therapy for B-CLL. Vδ2 targeting of Ph+ leukemia cells was demonstrated and immunotherapy experiments performed in a novel bioluminescent xenograft model of Ph+ leukemia. Despite the disappointing survival outcome of preclinical therapy experiments, decreased leukemia burden in some treated mice supports this line of investigation. Especially promising was detection of GDTc in the bone marrow of treated mice at the experimental endpoint, indicating GDTc localization to tumors, though whether migration was truly targeted was not addressed. The report of both a new GDTc expansion protocol and bioluminescent Ph+ leukemia model provides further tools with which we can contribute to the growing body of evidence supporting the development of GDTc as immunotherapeutic agents targeting leukemia.
| Abbreviations: | ||
| B-CLL | = | B-cell chronic lymphocytic leukemia |
| ConA | = | Concanavalin A |
| GDTc | = | gamma delta T cells |
| IV | = | intravenous |
| Ph+ | = | Philadelphia chromosome positive |
| TCR | = | T cell antigen receptor |
| Vδ1 | = | Vdelta1 gamma delta T cells |
| Vδ2 | = | Vgamma9Vdelta2 gamma delta T cells |
Acknowledgments
Thanks to Emeline J. Ribot, Christiane Mallet, Catherine McFadden and Conrad Siegers for critical reading of this manuscript.
References
- Ensslin AS, Formby B. Comparison of cytolytic and proliferative activities of human gamma delta and alpha beta T cells from peripheral blood against various human tumor cell lines. J Natl Cancer Inst 1991; 83:1564 - 9; http://dx.doi.org/10.1093/jnci/83.21.1564; PMID: 1683671
- Nakajima J, Murakawa T, Fukami T, Goto S, Kaneko T, Yoshida Y, et al. A phase I study of adoptive immunotherapy for recurrent non-small-cell lung cancer patients with autologous gammadelta T cells. Eur J Cardiothorac Surg 2010; 37:1191 - 7; http://dx.doi.org/10.1016/j.ejcts.2009.11.051; PMID: 20137969
- Lucas C, Ingoure S, Soule E, Faria B, Audibert F, Blery M, et al. IPH1101, the First Specific {gamma}{delta} T Cell Agonist, Shows Potent Immuno-Biological Efficacy in Low Grade Follicular Lymphoma Patients When Combined with Rituximab: Results From a Phase II Study. ASH Annual Meeting 2009; 114:583.
- Meraviglia S, Eberl M, Vermijlen D, Todaro M, Buccheri S, Cicero G, et al. In vivo manipulation of Vgamma9Vdelta2 T cells with zoledronate and low-dose interleukin-2 for immunotherapy of advanced breast cancer patients. Clin Exp Immunol 2010; 161:290 - 7; PMID: 20491785
- Lamb LS Jr., Musk P, Ye Z, van Rhee F, Geier SS, Tong JJ, et al. Human gammadelta(+) T lymphocytes have in vitro graft vs leukemia activity in the absence of an allogeneic response. Bone Marrow Transplant 2001; 27:601 - 6; http://dx.doi.org/10.1038/sj.bmt.1702830; PMID: 11319589
- Siegers GM, Dhamko H, Wang XH, Mathieson AM, Kosaka Y, Felizardo TC, et al. Human Vdelta1 gammadelta T cells expanded from peripheral blood exhibit specific cytotoxicity against B-cell chronic lymphocytic leukemia-derived cells. Cytotherapy 2011; 13:753 - 64
- Siegers GM, Felizardo TC, Mathieson AM, Kosaka Y, Wang XH, Medin JA, et al. Anti-leukemia activity of in vitro-expanded human gamma delta T cells in a xenogeneic Ph+ leukemia model. PLoS ONE 2011; 6:e16700; http://dx.doi.org/10.1371/journal.pone.0016700; PMID: 21304898
- Bartkowiak J, Kulczyck-Wojdala D, Blonski JZ, Robak T. Molecular diversity of gammadelta T cells in peripheral blood from patients with B-cell chronic lymphocytic leukaemia. Neoplasma 2002; 49:86 - 90; PMID: 12088111
- Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods 2003; 281:65 - 78; http://dx.doi.org/10.1016/S0022-1759(03)00265-5; PMID: 14580882
- Rothenfusser S, Buchwald A, Kock S, Ferrone S, Fisch P. Missing HLA class I expression on Daudi cells unveils cytotoxic and proliferative responses of human gammadelta T lymphocytes. Cell Immunol 2002; 215:32 - 44; http://dx.doi.org/10.1016/S0008-8749(02)00001-1; PMID: 12142034