Abstract
Repetitive infusions of ex vivo expanded NK cells induced antitumor T-cell responses in a metastatic lung cancer mouse model. These were further potentiated by Treg depletion. Thus the combination of NK cell-based immunotherapy with other treatment modalities in the direction of adaptive response enhancement might promote long lasting antitumor immunity.
NK cells are innate immune lymphocytes which primarily function to lyse virally infected cells and tumor cells and to regulate innate and adaptive immune responses through the production of cytokines and chemokines.Citation1,Citation2 The role of NK cells in tumor immunosurveillance and their potential for successful cancer immunotherapy strategies is currently established. The effectiveness of resting, shortly activated or ex vivo expanded autologous or allogeneic NK cell-based immunotherapies is explored in preclinical studies and clinical trials, for the treatment of hematological malignancies and solid tumors. The main issues addressed so far in the clinical application of NK cells referred to: (1) Overcoming inhibitory signals, either by using allogeneic NK cells or by blocking inhibitory receptors; (2) the number of cells to be infused; and (3) the in vivo persistence of the adoptively transferred NK cells in one single infusion through preconditioning lymphodepletion regimens and IL-2 co-administration.
The apparent goal of such approaches is to achieve a clinical response by eliminating as much as possible tumor cells by direct NK cell-mediated killing. However effective immunotherapy should elicit long lasting memory immune responses in order to prevent future relapse. Although NK cells have been demonstrated to possess memory against viruses,Citation3 there is no evidence yet that this could also apply to the diversity of tumor associated antigens (TAA).
Given that NK cells are part of an integral immune network where the function of each component population may affect, positively or negatively, the function of other immune cell populations, we explored the ability of NK cells to induce memory anti-tumor T cell immune responses in a metastatic lung cancer mouse model.
Considering that adoptively transferred NK cells would act, either indirectly, through cross-talk with dendritic cells (DC)Citation4 or directly, as antigen presenting cells (APC),Citation4,Citation5 to trigger priming of tumor-specific T cells (), we presumed that repeated administrations of ex vivo expanded NK cells would act as repeated anti-tumor “vaccines.” Indeed, after four weekly NK cell infusions we were able to detect memory systemic T cell responses against the syngeneic CT26 tumor cells, either by in vitro testing of splenic T cell reactivity or by rechallenging experimental animals with tumor cells in another site of the body.Citation6
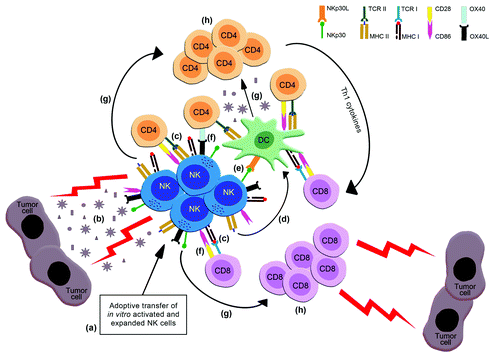
Figure 1. Adoptive transfer of ex vivo activated and expanded NK cells can act as tumor-specific active immunotherapy, inducing long lasting anti-tumor T cell responses. NK cells can be effectively activated and expanded in vitro by cytokines in combination with GCs and/or other “stress” signals (a). Adoptively transferred activated NK cells can lyse tumor cells thus leading to the release/ production of apoptotic bodies/TAAs (b). Such tumor-derived material will be processed and presented to CD4 and CD8 T cells (i) directly, by the activated NK cells which express MHC class I and class II molecules (c) or (ii) indirectly, through NK cell-mediated activation and polarization of DCs. This can be achieved by activated NK cell-produced cytokines (IFNγ, TNFα, GM-CSF) (d) or by direct cell-to-cell contact (i.e., NKp30-NKp30L) (e). Furthermore, activated NK cells expressing costimulatory molecules (e.g., CD86, OX40L, etc) can provide efficient costimulation to T cells (through CD28, OX40, etc) (f). The concerted action of cytokines produced by the activated NKs and DCs (IFNγ, IL-12) (g) will support Th1 responses. The effective priming and restimulation of T cells by NK cells and/or DCs will induce the proliferation and differentiation of CD4+ and CD8+ into effector/memory and central memory cells that can confer long lasting antitumor immunity (h). Improvement of these responses can be achieved through inhibition of immunomodulatory mechanisms (e.g., Treg cell and MDSC elimination/ inhibition, anti-CTLA4 or anti-PD1 targeting etc) as applied in other vaccination strategies (e.g., peptide vaccines, whole cell vaccines, DC-based vaccines etc.).
To further optimize these anti-tumor responses, we treated tumor-bearing animals with Ontak® (Denileukin Diftitox) to eliminate regulatory T cells (Treg), once, two days prior to the first NK cell administration. Given that activated NK cells are resistant to Treg-induced suppression, we hypothesized that elimination of Tregs prior to T cell priming would lead to more robust, long lasting immune responses. Indeed, under these conditions 50% of the mice were cured; splenic T cells produced more IFNγ and exhibited higher cytotoxicity and proliferation against the CT26 cells in vitro; furthermore the growth rate of distal secondary tumors was significantly delayed, compared with mice treated with NK cells alone.Citation6
Another important parameter to be considered when applying cell-based cancer immunotherapies is the ability of the adoptively transferred cells to home to the tumor sites. When considering solid tumors, the persistence of the adoptively transferred NK cells in the peripheral blood is not informative about their effectiveness to reach the tumor. This could explain the recently published data from a clinical trial of adoptive transfer of autologous NK cells in patients with metastatic melanoma or renal cell carcinoma: although the adoptively transferred NK cells appeared to persist in the peripheral circulation of patients for at least one week post transfer, and in some patients for several months, no clinical responses were observed.Citation7
In the case of our lung metastasis tumor model, we found that about 60% of the infused NK cells were present within the lungs of the tumor-bearing animals three days after their i.v. infusion and rapidly declined to 25% after one week. Given that activated NK cells are known to infiltrate the lung tissue within minutes of i.v. injection and can be retained by the tumor,Citation8 we can assume that only few NK cells remained in the circulation.
The NK cells used in our preclinical model were ex vivo expanded with IL-15 in the presence of hydrocortisone, a combination that as we have previously demonstrated, both in human and mice, significantly accelerates NK cell proliferation, rendering these cells more resistant to cytokine-induced cell death without compromising their functional potential.Citation6,Citation9,Citation10 Furthermore, NK cells expanded in the presence of glucocorticoids (GC) express high levels of CXCR3 and CXCR4,Citation6,Citation10 indicating their increased potential to home to the tumor sites.
Our data put forward an additional, so far unappreciated role for NK cells acting as potent “cellular vaccines” inducing and/or boosting endogenous adaptive antitumor responses. In this context, NK cell-based cellular therapy could be combined with active and passive immunotherapies as well as with other potential modalities of cancer therapy including chemotherapy, radiotherapy and biological therapies. This novel biological role ascribes NK cells a central position in the field of active cancer immunotherapies in the context of personalized medicine. The exploitation of the multi-talented NK cells against cancer is still in its infancy and needs to be quickly explored under a new perspective.
References
- Sutlu T, Alici E. Natural killer cell-based immunotherapy in cancer: current insights and future prospects. J Intern Med 2009; 266:154 - 81; http://dx.doi.org/10.1111/j.1365-2796.2009.02121.x; PMID: 19614820
- Terme M, Ullrich E, Delahaye NF, Chaput N, Zitvogel L. Natural killer cell-directed therapies: moving from unexpected results to successful strategies. Nat Immunol 2008; 9:486 - 94; http://dx.doi.org/10.1038/ni1580; PMID: 18425105
- Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, et al. Innate or adaptive immunity? The example of natural killer cells. Science 2011; 331:44 - 9; http://dx.doi.org/10.1126/science.1198687; PMID: 21212348
- Zingoni A, Sornasse T, Cocks BG, Tanaka Y, Santoni A, Lanier LL. NK cell regulation of T cell-mediated responses. Mol Immunol 2005; 42:451 - 4; http://dx.doi.org/10.1016/j.molimm.2004.07.025; PMID: 15607797
- Hanna J, Gonen-Gross T, Fitchett J, Rowe T, Daniels M, Arnon TI, et al. Novel APC-like properties of human NK cells directly regulate T cell activation. J Clin Invest 2004; 114:1612 - 23; PMID: 15578093
- Salagianni M, Lekka E, Moustaki A, Iliopoulou EG, Baxevanis CN, Papamichail M, et al. NK cell adoptive transfer combined with Ontak-mediated regulatory T cell elimination induces effective adaptive antitumor immune responses. J Immunol 2011; 186:3327 - 35; http://dx.doi.org/10.4049/jimmunol.1000652; PMID: 21317394
- Parkhurst MR, Riley JP, Dudley ME, Rosenberg SA. Adoptive transfer of autologous natural killer cells leads to high levels of circulating natural killer cells but does not mediate tumor regression. Clin Cancer Res 2011; In press http://dx.doi.org/10.1158/1078-0432.CCR-11-1347; PMID: 21844012
- Albertsson PA, Basse PH, Hokland M, Goldfarb RH, Nagelkerke JF, Nannmark U, et al. NK cells and the tumour microenvironment: implications for NK-cell function and anti-tumour activity. Trends Immunol 2003; 24:603 - 9; http://dx.doi.org/10.1016/j.it.2003.09.007; PMID: 14596885
- Perez SA, Mahaira LG, Demirtzoglou FJ, Sotiropoulou PA, Ioannidis P, Iliopoulou EG, et al. A potential role for hydrocortisone in the positive regulation of IL-15-activated NK-cell proliferation and survival. Blood 2005; 106:158 - 66; http://dx.doi.org/10.1182/blood-2004-08-3232; PMID: 15755904
- Moustaki A, Argyropoulos KV, Baxevanis CN, Papamichail M, Perez SA. Effect of the simultaneous administration of glucocorticoids and IL-15 on human NK cell phenotype, proliferation and function. Cancer Immunol Immunother 2011; 60:1683 - 95; http://dx.doi.org/10.1007/s00262-011-1067-6; PMID: 21706285