Abstract
Macrophage infiltration is a hallmark in the majority of solid tumors. Our studies demonstrated that macrophages that infiltrate human renal cells carcinoma (RCC) display markedly enhanced expression and activity of 15-lipoxygenase-2 (15-LOX2). Obtained data suggest that enhanced lipoxygenase activity in tumor-associated macrophages stimulates cancer inflammation and causes immune dysfunction.
Advanced, metastatic RCC disease is uniformly resistant to radiation and chemotherapy and it represents one of the most receptive cancers to immunotherapy.Citation1 However, the clinical response is weakened, mostly due to tumor-induced immune suppression that represents a major obstacle for successful cancer immunotherapy. Multiple immunosuppressive mechanisms have been identified in RCC that propagate conditions interfering with the development and activation of an effective anti-tumor response.Citation2,Citation3 Tumor microenvironment, which encompasses immune, stromal and vascular cells, exerts profound immunosuppressive and tolerogenic activity on antigen presenting as well as T effector cells by secretion of bioactive proteins and lipids. Together, these factors favor conditions that allow tumors to escape immune recognition and foster proliferative and metastatic potential of tumor cells.
Growing body of evidence suggests that cancer progression is facilitated by enhanced production of eicosanoids that promote cancer inflammation, immune suppression, angiogenesis and proliferation of malignant cells.Citation4,Citation5 Eicosanoids are enzymatic products of arachidonic acid (AA) and can be formed by cyclooxygenase (COX), lipoxygenase (LOX) or cytochrome P-450 epoxygenase. Importantly, eicosanoids strongly contribute to the immunoregulatory mechanisms and may control inflammation through regulation of cytokine and chemokine production. Arachidonate products of cycloxygenase and lipoxygenase pathways can be secreted in substantial amounts by both epithelial cancer cells and tumor-infiltrating inflammatory cells including tumor-associated macrophages and myeloid-derived suppressor cells.Citation6,Citation7 Numerous studies provided clinical and pharmacological evidence for involvement of COX2-PGE2 pathway in the pathogenesis of various cancers, including colon, lung, breast and bladder cancers. The other AA metabolizing enzymes (e.g., lipoxygenases) have drawn less attention for their potential roles in human tumor initiation and/or progression.
Lipoxygenases (LOXs) are iron-containing dioxygenases that catalyze formation of fatty acid hydroperoxides from polyunsaturated fatty acids.Citation8 The specific bioactivities of LOXs include hydroperoxidase, leukotriene synthase, lipoxin synthase and hepoxylin synthase activities. Four major types of mammalian LOXs were described: 5-LOX, 8-LOX, 12-LOX and 15-LOX. In humans, two distinct subtypes of 15-LOX exist: 15-LOX1 (encoded by 15ALOX gene) and 15-LOX2 (encoded by 15ALOXB gene). Arachidonic acid (AA) is the major substrate for 15-LOX2, whereas 15-LOX1 prefers linoleic acid as substrate but could also metabolize AA. 15(S)-HETE is the major metabolite of AA formed by 15-LOX2 and 15-LOX1.
Recently we have demonstrated that progression of human renal cell carcinoma is associated with enhanced expression and activity of 15-LOX2.Citation9 RCC tissues are frequently infiltrated with CD45+CD11b+CD68+HLA-DR+ tumor-associated macrophages (TAMs). TAMs seem to be central players in deregulated eicosanoid production observed in RCC because they secrete the highest levels of major 15-LOX2 arachidonate metabolite 15(S)-HETE and highly expressed 15-LOX2 at both gene and protein levels. Isolated TAMs produce substantial amounts of pro-inflammatory chemokine CCL2 and immunosuppressive cytokine IL-10. Co-incubation of TAMs with autologous T lymphocytes resulted in dramatic (4–10 fold) increase of IL-10 production. Remarkably, inhibition of lipoxygenase activity significantly reduced secretion of CCL2 and IL-10 by TAMs, and also prevented TAM-mediated induction of IL-10 in T cells. In addition, TAMs were able to induce tolerogenic transcription factor FOXP3 and inhibitory receptor CTLA-4 in T lymphocytes. However, the ability of TAMs to convert T cells into FOXP3+ T regulatory cells or induce CTLA-4 did not depend on 15-LOX2 activity. It would be important in further studies to delineate the mechanism by which TAMs induce FOXP3+ T regs in kidney cancer.
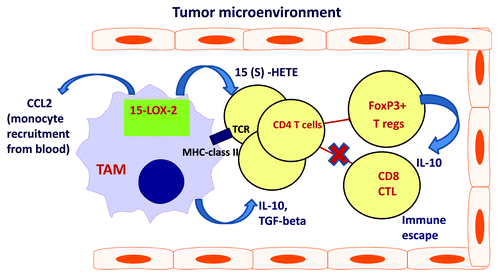
Cancer therapy and immunomodulatory therapy of RCC is less effective in treating large tumors partly due to immune suppression associated with advanced metastatic cancer disease. Our data suggest that tumor-associated macrophages play an important role in the development of immune suppression and T cell tolerance in human RCC through secretion of immunosuppressive factors and induction of immune unresponsiveness in T cells. We demonstrated that immune suppression in RCC is closely relates to deregulated eicosanoid metabolism in the tumor microenvironment. provides a schematic overview of the contribution 15-LOX2-expressing tumor-associated macrophages in immune dysfunction in patients with kidney cancer. This figure illustrates that macrophages infiltrating human RCC have a significant impact on cancer inflammation via CCL2-mediated recruitment of monocytes to the tumor siteCitation10 and inhibition of generation of anti-tumor immune response via secretion of IL-10 and also by conversion of T cells into T regs.
Figure 1. Tumor-associated macrophages contribute to the cancer immune suppression and cancer inflammation in RCC via enhanced 15-LOX2/15-S-HETE pathway. Enhanced 15-LOX2 activity and elevated levels of eicosanoids of RCC tumor-microenvironment enable increased production of pro-inflammatory chemokine CCL2. Elevated levels of CCL2 in tumor lead to migration of CCR2-expressing monocytes from peripheral blood to the tumor. In tumor tissue recruited monocytes differentiate in 15-LOX-expressing tumor-associated macrophages (TAMs). High 15-LOX2 expression/activity in TAMs results in elevated secretion of arachidonate metabolites and increased production of IL-10 and CCL2 by TAMs and T cells. In addition, TAMs efficiently convert T cells into FOXP3+ T regs. Together, elevated levels of IL-10 and T regs promote local immunosuppression and T cell tolerance in RCC tumor microenvironment.
Our results suggest that manipulation of deregulated metabolism of arachidonic acid and reduction of eicosanoid levels in tumor microenvironment represents an attractive approach to regulate both cancer-related inflammation and immune suppression in human cancers including RCC. Inhibition of 15-LOX2 activity could potentially synergize with existing RCC immunotherapy such as IL-2, IFNα or dendritic-based therapy thereby enhancing its therapeutic effect and improving overall survival of cancer patients.
References
- Atkins MB, Avigan DE, Bukowski RM, Childs RW, Dutcher JP, Eisen TG, et al. Innovations and challenges in renal cancer: consensus statement from the first international conference. Clin Cancer Res 2004; 10:6277S - 81S
- Deerwish IH, Tannebaum CS, Rayman PA, Finke JH. Mechanisms of immune dysfunction in renal cell carcinoma. Cancer Treat Res 2003; 116:29 - 51
- Kusmartsev S, Vieweg J. Enhancing efficacy of cancer vaccines in urologic oncology: new directions. Nature Reviews Urology 2009; 6:540 - 9
- Wang D, Dubois R. Eicosanoids and cancer. Nat Rev Cancer 2010; 10:181 - 93
- Greene ER, Huang S, Serhan CN, Panigrahy D. Regulation of inflammation in cancer by eicosanoids. Prostaglandins Other Lipid Mediat 2011; 96:27 - 36
- Eruslanov E, Daurkin I, Ortiz J, Vieweg J, Kusmartsev S. Pivotal Advance: Tumor-mediated induction of myeloid-derived suppressor cells and M2-polarized macrophages by altering intracellular PGE2 catabolism in myeloid cells. J Leukoc Biol 2010; 88:839 - 48
- Obermajer N, Muthuswamy R, Lesnock J, Edwards RP, Kalinski P. Positive feedback between PGE2 and COX2 redirects the differentiation of human dendritic cells towards stable myeloid-derived suppressor cells. Blood 2011; 118:5498 - 505
- Kuhn H, O’Donnel VB. Inflammation and immune regulation by 12/15 lipoxygenases. Prog Lipid Res 2006; 45:334 - 56
- Daurkin I, Eruslanov E, Stoffs T, Perrin GQ, Algood C, Gilbert SM, et al. Tumor-associated macrophages mediate immunosuppression in the renal cancer microenvironment by activating the 15-lipoxygenase-2 pathway. Cancer Res 2011; 71:6400 - 9
- Mantovani A, Schioppa T, Porta C, Allavena P, Sica A. Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev 2006; 25:315 - 22