Abstract
Developers of cancer immunotherapy have struggled for decades to achieve clinical success in using the patient’s immune system to treat cancer. In the absence of a defined development paradigm for immunotherapies, conventional criteria established for chemotherapy were applied to these agents. This article summarizes the recent lessons for development of agents in the immunotherapy space, describes the systematic creation of a new clinical development paradigm for cancer immunotherapies and integrates this paradigm with the emerging methodological framework for a new clinical sub-specialty of immuno-oncology, which was driven by the collaborative work between the Cancer Immunotherapy Consortium (CIC) of the Cancer Research Institute in the US and the Association for Cancer Immunotherapy (CIMT) in Europe. This new framework provides a better defined development path and a foundation for more reproducible success of future therapies.
Background
Immunotherapy of cancer has a history full of important discoveries that goes back to the late nineteenth century, when William B. Coley observed tumor regressions following the injection of a bacterial broth also known as Coley’s toxins into malignant lesions. This was followed by a series of discoveries but also draw-backs that have led to marked fluctuations in attitude toward cancer immunotherapy.Citation1,Citation2 Until recently, this field has not systematically assessed and integrated the available knowledge on the unique aspects of immune therapeutic approaches to establish a methodological framework for rational clinical development that enables reproducible clinical success.Citation3,Citation4 Such reproducible success is needed for the cancer immunotherapy field to improve the perception among stakeholders and rise as a recognized sub-specialty within oncology and human medicine.
Cancer immunotherapies, ranging from monoclonal antibodies to complex cellular vaccines, have long been considered as promising, and are expected to provide clinical benefit with focused or minimal toxicities. The fact that they have not yet delivered on that promise may be due to the incomplete scientific understanding of tumor immunology on one hand and the use of conventional development plans as defined for the distinctly different but more familiar chemotherapeutic agents on the other hand. Thus, some immunotherapy failures can probably be explained by their lack of efficacy, while others may be due to an inadequate approach to their development. Recently, Goldman and DeFrancesco pointed out that some reasons for developmental failures include “companies not doing their homework” and asked “what lessons from the list of failures will inform future practitioners in the field?”Citation3
Such lessons are now available after having been systematically defined over the past decade with the aim to improve the developmental path and enable success in the cancer immunotherapy space.Citation4-Citation6 Driven by the struggle of biotechnology companies and academic institutions devoted to immunotherapies, the Cancer Immunotherapy Consortium [CIC; formerly Cancer Vaccine Consortium, a program of the non-profit Cancer Research Institute (CRI)], which was founded for the advancement of the cancer immunotherapy field, began to create a systematic framework that would provide the knowledge and tools needed for their successful development. CIC formed a partnership with the Association for Cancer Immunotherapy (CIMT) in Europe, and, with broad contributions from the scientific and drug development communities developed the new framework that encompasses the following: a development paradigm for cancer immunotherapies, harmonized use of methods for measuring immune response as a foundation for immune biomarker development,Citation7,Citation8 improved study designsCitation5 and clinical endpoints,Citation9,Citation10 a publication framework for immune monitoring results from clinical trials,Citation11,Citation12 and scientific exchange and regulatory interactions to inform guidance document development by regulatory authorities.Citation13,Citation14
In the last three years the field - for the first time—saw the clinical proof of success for cancer immunotherapies achieved in controlled randomized Phase III trials meeting primary survival endpoints: the first regulatory approvals of a therapeutic cancer vaccine, sipuleucel-T (Provenge®) for hormone-refractory prostate cancer,Citation15 and for the T-cell potentiating monoclonal antibody anti-CTLA-4 (ipilimumab; Yervoy).Citation16,Citation17 With these historic milestones reached in two unrelated tumor entities and two distinct agents, immunotherapy is finding entry to the landscape of prescription medicines in oncology next to the existing approaches of chemotherapy, small molecule targeted therapies, radiation and surgery. The clinical development paths of both agents have contributed to define and to demonstrate the practical value of the new development paradigm.Citation4
Here we provide a perspective on the recent lessons in the immunotherapy space and summarize the emerging framework that promises to enable greater and more reproducible success and provide credibility for future development ().
Figure 1. The immuno-oncology framework provides a set of methodological improvements for the development of cancer immunotherapies, which are tailored to this class of therapies. Each component addresses a relevant piece of the drug development process and was evolved out of a community consensus approach through the immunotherapy organizations CIC and CIMT. The framework is expected to expand with ongoing evolution of the field.
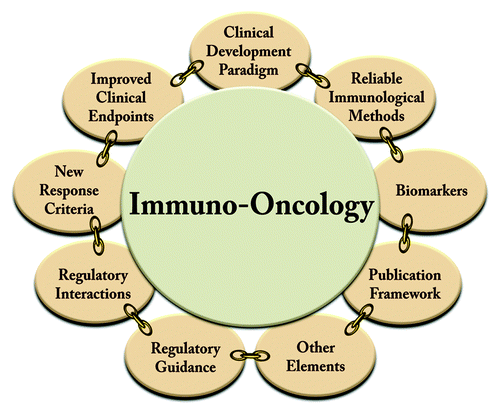
The Evolving Framework for Immuno-Oncology
Oncology, the clinical discipline of cancer therapy, has been an established medical specialty for several decades. Its hallmarks are the science of cancer biology as described by Hanahan and Weinberg;Citation18,Citation19 a recognized clinical development paradigm (based on observations with chemotherapy) for investigation of new therapies in Phase 1, 2 and 3 clinical trials; defined criteria for measuring therapeutic effects such as RECIST (Response Evaluation Criteria In Solid Tumors) or WHO (World Health Organization) criteria for solid tumors; understood kinetics of therapeutic effects; established standards for publication of new scientific data; and the availability of effective therapies paired with a clear understanding of their use. All this is embedded in a well-circumscribed community represented by organizations such as the American Society of Clinical Oncology (ASCO) and the European Society of Medical Oncology (ESMO). Together, these hallmarks create a framework of credibility in which patient care, scientific discovery, publication, clinical development and regulatory review can take place.
Despite clear evidence that the whole class of cancer immunotherapies has critical unique features that are different from those of the established classical therapeutic approaches in oncology, the field did not yet create an appropriate alternative methodological framework accommodating these class-specific characteristics. Rather, to minimize controversy and gain recognition in oncology, investigations of immunotherapies utilized the existing development pathway that was based on cytotoxic drugs. This ultimately may have contributed to many failures in development.
Between 2004 and today, CIC and CIMT filled this void by creating a systematic framework incorporating broad community knowledge and providing needed tools for successful development of immunotherapies ().
Table 1. The framework for immuno-oncology: Solutions for common challenges
Cornerstones of a New Development Paradigm for Cancer Immunotherapy
The process began with the proposal of a clinical development paradigm. In 2004, much knowledge around developmental problems and potential solutions existed in the field, however, there had been little consensus on how to uniformly utilize this knowledge and pull together a more comprehensive paradigm. CIC-CRI and another partner organization, the international Society for Biological Therapy of Cancer (iSBTc; now the Society for Immunotherapy of Cancer) formed a community-wide initiative known as the Cancer Vaccine Clinical Trial Working Group (CVCTWG), including key stakeholders from academia, the biotechnology and pharmaceutical industry and the US FDA to construct and recommend a paradigm for development of cancer vaccines and related immunotherapies. The proposed paradigm recognizes differences between chemotherapy and immunotherapy such as: (1) the optimal biologic dose is often not the maximum tolerated dose; (2) treatment effect is not proportionally linked to toxicity; (3) conventional pharmacokinetics may not determine dose and schedule; (4) anti-tumor response is not the sole predictor of survival; and (5) clinical effects can be delayed in time and can occur after tumor volume increase (often categorized as progression). The new paradigm breaks down the development process into proof-of-principle trials and efficacy trials, where efficacy trials are recommended to be randomized (Phase 2 and 3 trials). Further, it offers considerations for toxicity screening in early trials, concepts for measurement of biologic activity, use of immune response assays in clinical trials, dose and schedule investigation, decision points in development, trial design, improved clinical endpoints, and combination therapy. Besides the systematic approach to the developmental science, much value of this paradigm lies in the consensus between all main constituents involved with cancer immunotherapy development namely academicians, pharmaceutical/biotech industry and the US FDA.Citation5,Citation13
New Clinical Effects Requiring Improved Clinical Endpoints
Conventional therapies exert their effects by directly targeting tumor cells and typically induce measurable impact on tumor growth soon after administration or not at all. In contrast, therapies targeting the immune system will provoke anti-tumoral effects indirectly by first stimulating the immune system and second leading to a broader range of response kinetics including delayed effects (after increase of existing tumor lesions or appearance of new lesions possibly induced by immune infiltrates) or stabilization of tumor lesions.Citation10 The latter patterns appear to be more common than the conventional response. They likely reflect the interplay between the immune system and the tumor described as immunoediting.Citation20 The described response kinetics also affect downstream events such as survival.
Delayed effects and stabilization of lesions influence the standard efficacy endpoints of anti-tumor response and overall survival and are observed across different immunotherapies.Citation6,Citation9 In both cases, endpoints need to be adjusted to address the biology. For the survival endpoint, Kaplan-Meier curves from randomized immunotherapy trials may show a delayed separation after months, which directly influences the statistical power to determine treatment effects observed over the entire length of the curves.Citation21 Statistical models used in randomized trials with conventional therapies, where separation of curves occurs early after treatment began, typically assume a constant hazard ratio over time (proportional hazards). Alternative statistical models tailored to address the delayed separation of curves have to consider that all events prior to the separation do not contribute to the differentiation between study arms after the separation, thus leading to loss of statistical power. Such new statistical models need to compensate for this loss of power. They may split the hazard ratio into an early and a late component before and after the separation of curves.Citation9 Importantly, the loss of power and absence of early effects should be carefully considered when designing randomized trials with early interim and futility analyses.
Modified Response Criteria for Immunotherapies
Standard WHOCitation22 or RECISTCitation23 criteria to assess clinical activity of anti-cancer agents were defined to capture effects of cytotoxic agents using tumor shrinkage as their measure of activity. Due to the new biology described above, the response patterns of immunotherapy extend beyond those of chemotherapy, and may manifest after a period of stable disease or after initial tumor burden increase or appearance of new lesions, which may reflect infiltration of lymphocytes into the tumor.Citation10,Citation24 Many investigators have noted such patterns but did not systematically describe them due to lack of suitable response criteria.Citation25,Citation26 Principles for development of new response criteria were derived from community workshops.Citation5 Subsequent large data sets supporting the development of new response criteria were generated as part of the ipilimumab (anti-CLTLA-4) development program encompassing 487 advanced melanoma patients.Citation10 The resulting data characterized four patterns of response. (1) immediate response; (2) durable stable disease with possible slow decline in tumor burden; (3) response after tumor burden increase (possible lymphocyte infiltration); and (4) response in the presence of new lesions. The resulting response criteria, termed immune-related response criteria (irRC) are generally based on WHO and RECIST criteria, describe tumor burden as a continuous variable over time, account for new lesions in the overall tumor burden and ask for confirmation of progression similar to the established confirmation of response at a subsequent time point after first detection. Available data suggest that irRC identify patients with previously unrecognized benefit as indicated by favorable survival outcomes of patients with novel response patterns as compared with patients with progressive disease.Citation10 However, the irRC still are undergoing prospective validation.
Managing Data Variability in Immune Biomarker Development
As immunotherapy targets immune cells and not tumors, the monitoring of treatment-induced immune responses defines relevant parameters for elucidation of the therapeutic mode of action and the description of early biologic effects prior to reaching clinical endpoints. Consequently, immunological monitoring using reliable and reproducible assays can enhance clinical development by providing information on (1) determining whether an immune intervention hit the biological target; (2) defining dose and schedule for the intervention; (3) measuring synergistic effects for therapeutic combinations; (4) defining study populations; (5) measuring therapeutic effects as biological activity; or (6) predicting clinical outcomes as surrogates for clinical benefit.Citation27
Commonly used cellular immune assays to determine the function, phenotype and frequency of antigen-specific T cells and other immune effectors (e.g., ELISPOT, intracellular cytokine staining, and HLA-peptide multimer staining) bear high data variability.Citation11 This variability has contributed to the field’s challenge to develop biomarkers for the above clinical applications. A possible strategy to reduce data variability observed across institutions can now be proposed. Immune response assay harmonization, which provides an external quality-control mechanism and guidance for assay conduct was undertaken by CIC and CIMT in the context large international proficiency panel programs.
As a result of these proficiency panels, in which more than 120 laboratories from academia, the biotechnology and the pharmaceutical industry from all over the world are participating, assay harmonization has been proposed as a solution (4) to manage data variability. Assay harmonization can increase assay performance within laboratories and decrease variation of results generated across institutionsCitation28-Citation33 thereby offering a tool to improve data reliability for immune monitoring and enhance clinical development of immune therapies at any stage of assay evolution.Citation8,Citation34 Harmonization of assay conduct reminds of the successful initiatives of ICH-GCP for clinical protocols.Citation35 The broad usage of assay harmonization may bring immune monitoring to the forefront of immune biomarker development, support a better understanding of therapeutic modes of action and guide decision making in clinical development.Citation36
Increasing Consistency in Reporting of Immune Monitoring Results
Variability is not only limited to data resulting from immune monitoring experiments. In addition, there is wide variability of presentation of methods and results in scientific publications. Notably, as of today, many publications of T-cell assay experiments lack information on some or many of the critical variables known to impact on assay performance thus not allowing the reader to fully understand or reproduce the experiment. A publication framework defining a minimum set of critical information of assay reports would provide greater transparency to the scientific community about what experiment was done under which conditions and with which results. Based on the concept of Minimum Information About Biological and Biomedical Investigations (MIBBI),Citation37 which created such a mechanism over the last decade for more than 30 biological assays such as DNA microarrays, RNAi experiments or cellular assays, CIC and CIMT started the Minimal Information About T-Cell Assays (MIATA) project.Citation11 MIATA aims to establish a framework for publication of T cell assay results generated in clinical trials. The MIATA proposal is based on an extensive community-wide vetting process over approximately 2 y incorporating the expertise and concerns of more than 120 individuals from all areas of clinical immunology and is aiming for broad acceptance.Citation12 The final version of MIATA is currently being prepared and will become available in 2012.
Integration and Distribution of Key Scientific Knowledge
Knowledge growth is exponential in most scientific disciplines. However, filtration of the relevant information to facilitate practical progress is not straightforward. To achieve this for the cancer immunotherapy space, CIC (www.cancerresearch.org/consortium) and CIMT (www.cimt.eu) have adopted a simple and focused process to address challenges of the field in a one-at-a-time approach through workshops and annual scientific meetings. Both networks are open communities with wide participation among key stakeholders. One guiding principle is to foster the exchange of scientific information among colleagues active in basic science, clinical translation, industrial development and regulatory affairs to initiate field-spanning interactions. By bringing together the knowledge of different stakeholders from this still fragmented field, an integrative platform is steadily developing with synergistic effects that can only be reached through collaboration. Another dimension of knowledge transfer was reached by the formation of a trans-Atlantic collaboration between both associations leading to cross-fertilization and an avoidance of redundancy. Continued community participation and contribution of knowledge is a cornerstone of this process to address further challenges of the field and expand the framework for immuno-oncology.
Regulatory Guidance
The described science and new concepts for immunotherapy development evolved under CIC and CIMT auspices over several years and with the participation of all major stakeholders from academia, biotechnology and pharmaceutical industries, and the US FDA. In 2007, the FDA hosted a workshop where these topics were reviewed. Subsequently, the FDA published a draft regulatory guidance on “Clinical Considerations for Therapeutic Cancer Vaccines”Citation13 where many of these topics were included. The FDA draft document went through a public consultation period and has become available in its final version in late 2011. Recently, the European Medicines Agency (EMA) issued a concept paper to stimulate public feedback on a proposed revision of the guidance on “evaluation of anticancer medicinal products in man.” Notably, the concept paper is specifically addressing clinical endpoints for biologics and aims to include a section on cancer vaccines.Citation14 As the regulatory landscape is constantly evolving, CIMT has formally initiated a working group focusing on regulatory research that is screening for new draft guidance documents undergoing public consultations. The working group collects expert opinions among the network’s members and integrates all comments to one consolidated response representing the expertise of the community. Similarly, the CIC Executive Committee offers responses to regulatory guidance documents. CIC and CIMT aim for integration of both positions. This process enables the community to speak with a more uniform voice and makes it easier for officials at FDA and EMA to review and assess community positions.
Anti-CTLA-4 Antibody Development: A Clinical Case Study
The importance of the new immunotherapy paradigm is illustrated through the example of anti-CTLA-4 antibody development.Citation4,Citation17 Clinical investigation of anti-CTLA-4 antibodies started at the biotechnology company Medarex in 2000 with Phase 1 and 2 trials indicating close to 10% response rates as a signal of clinical activity in patients with advanced melanoma. Interest from big pharma for developing anti-CTLA-4 blocking antibodies led to independent licensing deals with Pfizer and Bristol-Myers Squibb (BMS) for isoforms of the antibody and two parallel development programs in advanced melanoma with tremelimumab (Pfizer) and ipilimumab (BMS), respectively. Both programs initially used chemotherapy criteria to guide their development choices.Citation4 As per its design, the tremelimumab program conducted an early interim analysis with conventional futility criteria for survival in its Phase 3 study and could not observe a survival improvement. Consequently, the Phase 3 study was terminated for futility as per Data Monitoring Committee recommendations.Citation38 Two years later, extended follow-up on the study population revealed a separation of survival curves.Citation39
Through the interaction of the ipilimumab program with the CIC efforts to create a new immunotherapy paradigm, the scientific approach for ipilimumab evolved and led to the change of the primary endpoint for both of its Phase 3 studies in advanced melanoma to overall survival with no early interim analyses, which could mislead the survival assessment.Citation4,Citation9,Citation16,Citation17 The final analysis of survival of two Phase 3 studies demonstrated improved survival (HR 0.66 and HR 0.72, respectively) and supported the approval for patients with pretreated metastatic melanoma. Based on the understanding of immunotherapy development BMS acquired Medarex in 2009 in a transaction valued $2.4 billion and is now developing a pipeline of immuno-oncology agents resulting from the acquisition.Citation40
The development paths for ipilimumab and tremelimumab and their respective results illustrate the importance of the science-driven clinical development paradigm for immunotherapies and of collaboration across various constituents to direct scientific progress. These observations also suggest that the prospective application of the new paradigm may help avoid critical pitfalls for future immunotherapy programs.
Conclusions
Our scientific knowledge of tumor immunology has steadily evolved,Citation41 and many cancer immunotherapy technologies have entered clinical trials.Citation3,Citation6,Citation42 An obvious weak spot for development has been the absence of a tailored clinical development paradigm for immunotherapies that distinguishes it from the widely used chemotherapy paradigm.
Over the past decade we have started to systematically address the unique characteristics of immunotherapeutic agents in clinical trials and developed a methodological framework to enable reproducible development.Citation4 This ranges from a defined immunotherapy development paradigm, improved clinical endpoints, harmonization concepts for immune monitoring to support immunological biomarker development, and minimal information for publication to enhance interpretability and reproducibility, as well as regulatory guidance. This new framework may provide for reproducible and likely more successful development of cancer immunotherapies and lays the foundation for the new clinical sub-specialty of immuno-oncology (). The framework may evolve with the growing field.
Acknowledgments
We thank all participants of the workshops and community-wide initiatives conducted by CIC and CIMT for the contribution of knowledge to this evolving framework.
References
- Lesterhuis WJ, Haanen JB, Punt CJ. Cancer immunotherapy--revisited. Nat Rev Drug Discov 2011; 10:591 - 600; http://dx.doi.org/10.1038/nrd3500; PMID: 21804596
- Parish CR. Cancer immunotherapy: the past, the present and the future. Immunol Cell Biol 2003; 81:106 - 13; http://dx.doi.org/10.1046/j.0818-9641.2003.01151.x; PMID: 12631233
- Goldman B, DeFrancesco L. The cancer vaccine roller coaster. Nat Biotechnol 2009; 27:129 - 39; http://dx.doi.org/10.1038/nbt0209-129; PMID: 19204689
- Hoos A, Britten CM, Huber C, O’Donnell-Tormey J. A methodological framework to enhance the clinical success of cancer immunotherapy. Nat Biotechnol 2011; 29:867 - 70; http://dx.doi.org/10.1038/nbt.2000; PMID: 21997622
- Hoos A, Parmiani G, Hege K, Sznol M, Loibner H, Eggermont A, et al. A clinical development paradigm for cancer vaccines and related biologics. J Immunother 1997; 30:1 - 15
- Finke LH, Wentworth K, Blumenstein B, Rudolph NS, Levitsky H, Hoos A. Lessons from randomized phase III studies with active cancer immunotherapies--outcomes from the 2006 meeting of the Cancer Vaccine Consortium (CVC). Vaccine 2007; 25:Suppl 2 B97 - 109; http://dx.doi.org/10.1016/j.vaccine.2007.06.067; PMID: 17916465
- Britten CM, Janetzki S, van der Burg SH, Gouttefangeas C, Hoos A. Toward the harmonization of immune monitoring in clinical trials: quo vadis?. Cancer Immunol Immunother 2008; 57:285 - 8; http://dx.doi.org/10.1007/s00262-007-0379-z; PMID: 17721782
- van der Burg SH, Kalos M, Gouttefangeas C, Janetzki S, Ottensmeier C, Welters MJ, et al.. Harmonization of immune biomarker assays for clinical studies. Sci Transl Med 2011; 3:108ps44
- Hoos A, Eggermont AM, Janetzki S, Hodi FS, Ibrahim R, Anderson A, et al. Improved endpoints for cancer immunotherapy trials. J Natl Cancer Inst 2010; 102:1388 - 97; http://dx.doi.org/10.1093/jnci/djq310; PMID: 20826737
- Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbe´ C, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 2009; 15:7412 - 20; http://dx.doi.org/10.1158/1078-0432.CCR-09-1624; PMID: 19934295
- Janetzki S, Britten CM, Kalos M, Levitsky HI, Maecker HT, Melief CJ, et al. “MIATA”-minimal information about T cell assays. Immunity 2009; 31:527 - 8; http://dx.doi.org/10.1016/j.immuni.2009.09.007; PMID: 19833080
- Britten CM, Janetzki S, van der Burg SH, Huber C, Kalos M, Levitsky HI, et al. Minimal information about T cell assays: the process of reaching the community of T cell immunologists in cancer and beyond. Cancer Immunol Immunother 2011; 60:15 - 22; http://dx.doi.org/10.1007/s00262-010-0940-z; PMID: 21080166
- Guidance for industry: Clinical Considerations for Therapeutic Cancer Vaccines. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Biologics Evaluation and Research, September 2009. http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/UCM182826.pdf.
- Concept paper on the need to revise the guideline on the evaluation of anticancer medicinal products in man. European Medicines Agency. July 2010. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/09/WC500096730.pdf.
- Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al, IMPACT Study Investigators. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010; 363:411 - 22; http://dx.doi.org/10.1056/NEJMoa1001294; PMID: 20818862
- Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711 - 23; http://dx.doi.org/10.1056/NEJMoa1003466; PMID: 20525992
- Hoos A, Ibrahim R, Korman A, Abdallah K, Berman D, Shahabi V, et al. Development of ipilimumab: contribution to a new paradigm for cancer immunotherapy. Semin Oncol 2010; 37:533 - 46; http://dx.doi.org/10.1053/j.seminoncol.2010.09.015; PMID: 21074069
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000; 100:57 - 70; http://dx.doi.org/10.1016/S0092-8674(00)81683-9; PMID: 10647931
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646 - 74; http://dx.doi.org/10.1016/j.cell.2011.02.013; PMID: 21376230
- Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 2002; 3:991 - 8; http://dx.doi.org/10.1038/ni1102-991; PMID: 12407406
- Fine GD. Consequences of Delayed Treatment Effects on Analysis of Time-to-Event Endpoints. Drug Inf J 2007; 41:535 - 9
- WHO handbook for reporting results of cancer treatment. Geneva (Switzerland): World Health Organization Offset Publication No. 48, 1979.
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45:228 - 47; http://dx.doi.org/10.1016/j.ejca.2008.10.026; PMID: 19097774
- Ribas A, Chmielowski B, Glaspy JA. Do we need a different set of response assessment criteria for tumor immunotherapy?. Clin Cancer Res 2009; 15:7116 - 8; http://dx.doi.org/10.1158/1078-0432.CCR-09-2376; PMID: 19934296
- Kruit WH, van Ojik HH, Brichard VG, Escudier B, Dorval T, Dre´no B, et al. Phase 1/2 study of subcutaneous and intradermal immunization with a recombinant MAGE-3 protein in patients with detectable metastatic melanoma. Int J Cancer 2005; 117:596 - 604; http://dx.doi.org/10.1002/ijc.21264; PMID: 15945101
- van Baren N, Bonnet MC, Dre´no B, Khammari A, Dorval T, Piperno-Neumann S, et al. Tumoral and immunologic response after vaccination of melanoma patients with an ALVAC virus encoding MAGE antigens recognized by T cells. J Clin Oncol 2005; 23:9008 - 21; http://dx.doi.org/10.1200/JCO.2005.08.375; PMID: 16061912
- Wagner JA. Overview of biomarkers and surrogate endpoints in drug development. Dis Markers 2002; 18:41 - 6; PMID: 12364809
- Janetzki S, Panageas KS, Ben-Porat L, Boyer J, Britten CM, Clay TM, et al, Elispot Proficiency Panel of the CVC Immune Assay Working Group. Results and harmonization guidelines from two large-scale international Elispot proficiency panels conducted by the Cancer Vaccine Consortium (CVC/SVI). Cancer Immunol Immunother 2008; 57:303 - 15; http://dx.doi.org/10.1007/s00262-007-0380-6; PMID: 17721781
- Britten CM, Gouttefangeas C, Welters MJ, Pawelec G, Koch S, Ottensmeier C, et al. The CIMT-monitoring panel: a two-step approach to harmonize the enumeration of antigen-specific CD8+ T lymphocytes by structural and functional assays. Cancer Immunol Immunother 2008; 57:289 - 302; http://dx.doi.org/10.1007/s00262-007-0378-0; PMID: 17721783
- Britten CM, Janetzki S, Ben-Porat L, Clay TM, Kalos M, Maecker H, et al, HLA-peptide Multimer Proficiency Panel of the CVC-CRI Immune Assay Working Group. Harmonization guidelines for HLA-peptide multimer assays derived from results of a large scale international proficiency panel of the Cancer Vaccine Consortium. Cancer Immunol Immunother 2009; 58:1701 - 13; http://dx.doi.org/10.1007/s00262-009-0681-z; PMID: 19259668
- Attig S, Price L, Janetzki S, Kalos M, Pride M, McNeil L, et al, CRI-CIC Assay Working Group. A critical assessment for the value of markers to gate-out undesired events in HLA-peptide multimer staining protocols. J Transl Med 2011; 9:108; http://dx.doi.org/10.1186/1479-5876-9-108; PMID: 21745365
- Mander A, Gouttefangeas C, Ottensmeier C, Welters MJ, Low L, van der Burg SH, et al. Serum is not required for ex vivo IFN-gamma ELISPOT: a collaborative study of different protocols from the European CIMT Immunoguiding Program. Cancer Immunol Immunother 2010; 59:619 - 27; http://dx.doi.org/10.1007/s00262-009-0814-4; PMID: 20052465
- Moodie Z, Price L, Gouttefangeas C, Mander A, Janetzki S, Löwer M, et al. Response definition criteria for ELISPOT assays revisited. Cancer Immunol Immunother 2010; 59:1489 - 501; http://dx.doi.org/10.1007/s00262-010-0875-4; PMID: 20549207
- Janetzki S, Britten CM. The impact of harmonization on ELISPOT assay performance. Methods Mol Biol 2012; 792:25 - 36; http://dx.doi.org/10.1007/978-1-61779-325-7_2; PMID: 21956498
- Ich harmonised tripartite guideline: guideline for good clinical practice. E6(R1). 1996. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6_R1/Step4/E6_R1__Guideline.pdf.
- van der Burg SH. Therapeutic vaccines in cancer: moving from immunomonitoring to immunoguiding. Expert Rev Vaccines 2008; 7:1 - 5; http://dx.doi.org/10.1586/14760584.7.1.1; PMID: 18251686
- Taylor CF, Field D, Sansone SA, Aerts J, Apweiler R, Ashburner M, et al. Promoting coherent minimum reporting guidelines for biological and biomedical investigations: the MIBBI project. Nat Biotechnol 2008; 26:889 - 96; http://dx.doi.org/10.1038/nbt.1411; PMID: 18688244
- Ribas A, Hauschild A, Kefford R, Punt CJA, Haanen JB. Phase III open-label, randomized, cmparative study of tremelimumab (CP-675,206) and chemotherapy (temozolomide [TMZ] or dacarbazine [DTIC]) in patients with advanced melanoma. American Society of Clinical Oncology, Annual Meeting 2008, abstract LBA 9011.
- Marshall M, Ribas A, Huang B. Evaluation of baseline serum C-reactive protein and benefit from tremelimumab compared to chemotherapy in first-line melanoma. American Society of Clinical Oncology, Annual Meeting, abstract 2609.
- BMS press release: Bristol-Myers Squibb to Acquire Medarex. July 22, 2009. http://www.bms.com/news/press_releases/pages/default.aspx.
- Finn OJ. Cancer immunology. N Engl J Med 2008; 358:2704 - 15; http://dx.doi.org/10.1056/NEJMra072739; PMID: 18565863
- Ribas A, Butterfield LH, Glaspy JA, Economou JS. Current developments in cancer vaccines and cellular immunotherapy. J Clin Oncol 2003; 21:2415 - 32; http://dx.doi.org/10.1200/JCO.2003.06.041; PMID: 12805342