Abstract
Antitumor effect of PolyI:C (a viral dsRNA analog) has been attributed to dendritic cell (DC)-maturation activity, that drives antitumor NK cells, DC cross-presentation, cytotoxic T lymphocytes and many IFN-inducible genes. According to a recent paper, tumor-infiltrating M2 macrophages are found to become an additional antitumor effector through polyI:C response.
Interferon (IFN), now categorized as type I, was discovered by Isaacs and Lindeman in 1957. Soon after their discovery, it was expected to be a fascinating medicine opposing to virus infection and cancer development. Type I IFN inducing activity was assigned to the signature of double-stranded RNA generated from viruses, and its synthetic analog, polyI:C, was confirmed to serve as an effective inducer of type I IFN. Talmadge et al.,Citation1 showed that polyI:C mixed with polyL Lysine and methylcellulose (polyI:CLC) effected dramatic regression of syngenic implant tumors in mice. They suggested this reagent might be applied to antitumor therapy. In line with these reports, there have been many reports indicating that spontaneous tumor regression sometimes occurrs in cancer patients when they are exposed to viruses or viral vectors.
PolyI:C induces type I IFN and inflammatory cytokines. In addition, it may contribute to raising cellular immunity. According to recent progress in pattern recognition of innate immunity, polyI:C is a ligand for multiple receptors, including PKR, RIG-I, MDA5 and TLR3.Citation2 Virus replication usually amplifies dsRNA production inside the cytoplasm of affected cells and stimulates the cytoplasmic RNA sensors. In contrast, TLR3 is activated when dsRNA generated in infected cells is released and internalized into the endosome of bystander phagocytes,Citation2 such as dendritic cells (DC) and macrophages. dsRNA is delivered through a unique pathway involving Raftlin,Citation3 then the endosomal TLR3 passes the signal to the adaptor TICAM-1.Citation2 The multiple functionality of polyI:C may reflect its divergent receptor usage, and knockout mouse (KO) studies have therefore been indispensable for determination of the role of each receptor in antitumor immunity.
In mouse models, growth retardation of syngenic implanted tumor has been reportedly observed by administration of polyI:C, which is now attributable to liberated type I IFN and maturation of DC, that drives NK and killer T cells.Citation4,Citation5 The mechanisms whereby these effector cells are introduced by dsRNA are being elucidated on a molecular level: the TLR3/TICAM-1 pathway for dsRNA recognition in DC is involved in effector driving. In a recent paper, Shime et al., additionally identified the third antitumor effector induced by ip polyI:C administration.Citation6 PolyI:C acted on tumor-infiltrating macrophages and induced tumor growth retardation in some tumor species. Administration of polyI:C rapidly (< 12 h) led to tumor hemorrhagic necrosis followed by tumor regression. The results appear to resemble an earlier report by Old’s group on the TNF-α-mediated fibrosarcoma regression.Citation7 In fact, TNF-α participated in hemorrhagic necrosis in this case also. Shime et al., applied KO mice models for analyzing the signaling pathway by which the polyI:C-derived tumor regression occurs. Ultimately, their conclusion was that tumor-infiltrating macrophages (Mf) characterized by CD11b+/F4/80+/Gr-1low markers with sustaining tumor-supporting phenotype, M2, serves as a target for polyI:C and changes their properties to antitumor, M1-like, behaving like a tumoricidal effector. In these Mf, TLR3/TICAM-1 pathway, but not the IPS-1 pathway, is also mandatory for TNF-α production and tumor regression. Indeed, the marker profile of the Mf was similar to those reported as M2 Mf or tumor-associated Mf (TAM). It is notable that they have high expression levels of TLR3. Hence, the polyI:C tumor growth retardation is mechanically multifarious and involves TNF-α hemorrhagic necrosis.
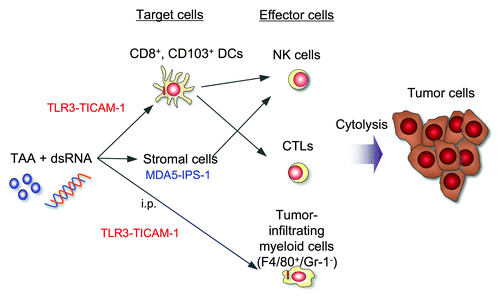
TLR3 is highly expressed in CD8+ splenic DC and CD103+ non-lymphoid DC in mice,Citation8 and they are strong inducers for cross-priming of CD8 T cells,Citation5,Citation8 namely cytotoxic T lymphocytes (CTL). TLR3-positive bone marrow-derived DC also reportedly induce type I IFN and potent antitumor NK cell activity.Citation4 Thus, polyI:C functions through TLR3+ myeloid cells to facilitates antitumor cellular immunity encompassing at least three distinct routes, NK cell activation, CTL proliferation and conversion of TAM to an tumoricidal effector (). Hence, the Janeway/Medzhitov conceptCitation9 may be adaptable to tumor immunology that pattern recognition receptor (PRR) stimulation by a specific ligand triggers innate immune response and facilitates establishment of the cellular immune system.
Figure 1. PolyI:C induces three antitumor effectors via different routes. Antitumor activity of polyI:C against tumor cells are assessed in mouse tumor-implant models. A unique point in this review is the third pathway where tumor-infiltrating myeloid cells are involved, effectively damages Lewis Lung carcinoma cells. This tumoricidal activity is mediated by the TICAM-1 pathway in the myeloid cells, and attributed to TNF-α. Although polyI:C is i.p. administered, it acts on tumor-infiltrating Mf and converts them to antitumor effectors.
A tantalizing reagent for successful peptide vaccine therapy against cancer using tumor-associated antigens (TAA) with CD4/CD8 epitopes is adjuvant. Nevertheless, polyI:C therapeutic use has been very restricted in patients. This is because polyI:C has severe side effects, enterocolitis, arthralgia, fever, erythema and sometimes life-threatening hypotonic shock, which have prevented the clinical use of this dsRNA analog. However, a recent study reported that polyI:CLC is applicable to humans, although robust erythema and cytokine upregulation in serum are usually accompanied as side effects with expected therapeutic potential.Citation10 Dr. Steinman, having won the Nobel prize, proposed a polyI:C/TAA therapy for cancer patients if the TAA is identified in each case of the patients. Shime’s data confirmed this issue and further clarified the importance of the TICAM-1 pathway in triggering induction of antitumor Mf in addtion to NK cells and CTL.Citation7 These sequential studies, together with the direct apoptotic effect of polyI:C on tumor cells, reinforce the need to establish a safer RNA derivative for human immunotherapy in the future.
References
- Talmadge JE, Adams J, Phillips H, Collins M, Lenz B, Schneider M, et al. Immunomodulatory effects in mice of polyinosinic-polycytidylic acid complexed with poly-L-lysine and carboxymethylcellulose. Cancer Res 1985; 45:1058 - 65; PMID: 3155990
- Matsumoto M, Oshiumi H, Seya T. Antiviral responses induced by the TLR3 pathway. Rev Med Virol 2011; 21:67 - 77; http://dx.doi.org/10.1002/rmv.680; PMID: 21312311
- Watanabe A, Tatematsu M, Saeki K, Shibata S, Shime H, Yoshimura A, et al. Raftlin is involved in the nucleocapture complex to induce poly(I:C)-mediated TLR3 activation. J Biol Chem 2011; 286:10702 - 11; http://dx.doi.org/10.1074/jbc.M110.185793; PMID: 21266579
- Ebihara T, Azuma M, Oshiumi H, Kasamatsu J, Iwabuchi K, Matsumoto K, et al. Identification of a polyI:C-inducible membrane protein that participates in dendritic cell-mediated natural killer cell activation. J Exp Med 2010; 207:2675 - 87; http://dx.doi.org/10.1084/jem.20091573; PMID: 21059856
- Azuma M, Ebihara T, Oshiumi H, Matsumoto M, Seya T. Cross-priming for antitumor CTL induced by soluble Ag + polyI:C depends on the TICAM-1 pathway in mouse CD11c+/CD8α+ dendritic cells. OncoImmunol 2012; In press
- Shime H, Matsumoto M, Oshiumi H, Tanaka S, Nakane A, Iwakura Y, et al. Toll-like receptor 3 signaling converts tumor-supporting myeloid cells to tumoricidal effectors. Proc Natl Acad Sci U S A 2012; 109:2066 - 71; http://dx.doi.org/10.1073/pnas.1113099109; PMID: 22308357
- Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A 1975; 72:3666 - 70; http://dx.doi.org/10.1073/pnas.72.9.3666; PMID: 1103152
- Jelinek I, Leonard JN, Price GE, Brown KN, Meyer-Manlapat A, Goldsmith PK, et al. TLR3-specific double-stranded RNA oligonucleotide adjuvants induce dendritic cell cross-presentation, CTL responses, and antiviral protection. J Immunol 2011; 186:2422 - 9; http://dx.doi.org/10.4049/jimmunol.1002845; PMID: 21242525
- Medzhitov R, Preston-Hurlburt P, Janeway CA Jr.. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 1997; 388:394 - 7; http://dx.doi.org/10.1038/41131; PMID: 9237759
- Caskey M, Lefebvre F, Filali-Mouhim A, Cameron MJ, Goulet JP, Haddad EK, et al. Synthetic double-stranded RNA induces innate immune responses similar to a live viral vaccine in humans. J Exp Med 2011; 208:2357 - 66; http://dx.doi.org/10.1084/jem.20111171; PMID: 22065672