Abstract
Our own experience and a thorough literature review suggest that interferon α (IFNα) should be reconsidered for the treatment of acute myeloid leukemia patients. Most likely, the success of such treatment depends on the achievement of high serum levels of IFNα for several months, which can be obtained by using pegylated IFNα.
Starting in 1966 (with a peak in the 1980s and 1990s), the results of no less than 34 clinical studies on the antineoplastic activity of interferon α (IFNα) in acute myeloid leukemia (AML) patients have been published.Citation1-Citation3 IFNα has been tested in three different therapeutic settings: (1) for the induction of AML remission, (2) as a salvage therapy for the treatment of patients relapsing upon hematopoietic stem cell transplantation (HSCT), and (3) as a post-remission strategy to prevent recurrence.Citation1 Although objective clinical responses were observed in all such settings, reported clinical outcomes are considerably heterogeneous, probably linked to similarly heterogeneous study designs. As a consequence, firm conclusions about the therapeutic role of IFNα could not be made, explaining why IFNα did not become a standard treatment option for AML patients.Citation1
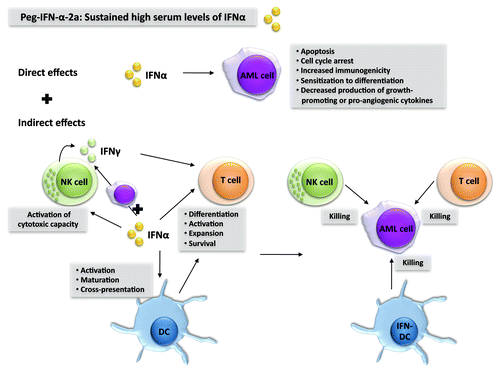
Nevertheless, there is a biological rationale for the use of type I IFN (IFNα or IFNβ) to treat AML ().Citation1 First, type I IFN exerts direct antitumor effects on AML cells by multiple mechanisms, as it (1) limits the secretion of growth-promoting cytokines, (2) stimulates apoptosis, (3) inhibits cell proliferation, and (4) increases the immunogenicity of AML cells. Second, type I IFN exerts indirect antitumor effects by activating dendritic cells (DCs), natural killer (NK) cells and T cells, three cell types that play a major role in antitumor immune responses. In this context, a recent report has shown that type I IFN is critical to the initiation of antitumor immunity through direct actions on cross-presenting DCs.Citation4 In addition, IFNα, similar to interleukin-15,Citation5 can induce DCs to become killer cells and hence exert direct cytotoxic activity against AML cells.Citation6,Citation7
Figure 1. Biological rationale behind the use of interferon α to treat acute myeloid leukemia. Interferon α (IFNα) can exert direct as well as indirect anticancer effects against acute myeloid leukemia (AML) cells. Indirect antineoplastic effects stem as IFNα stimulates dendritic cells (DCs), natural killer (NK) cells and T cells to exert antileukemic functions. By using pegylated (peg)-IFNα, sustained high serum levels of IFNα can be achieved, which is probably a prerequisite for the success of such an immunotherapeutic regimen against AML.
Why has IFNα failed to show consistent clinical benefit in AML patients, in spite of the conceptual background supporting its potential efficacy? A preclinical study performed by Benjamin et al.Citation8 identified what could have made the difference between treatment success or failure. Thus, high IFN serum levels (at least 3000 IU/mL) during a prolonged period seem to be a prerequisite for treatment success in AML.Citation1,Citation8 According to this study, the therapeutic potential of IFNα can perhaps be unlocked by the use of long-acting IFN preparations, such as IFNα conjugated to a polyethylene glycol moiety (pegylated-IFNα). Such modified IFNα preparations have an improved pharmacokinetic profile that allows for the protracted maintenance of high and stable serum IFNα levels.
We tested pegylated IFNα-2a in a patient affected by AML secondary to myelofibrosis who turned down chemotherapy and HSCT.Citation2 After 5 mo of treatment with at least 180 µg pegylated IFNα-2a per week, bone marrow analyses showed complete AML remission. This remission lasted for more than 3 y and the patient is still alive after 4 y, far longer than the expected 6-mo survival after diagnosis.Citation9 Currently, high doses of pegylated IFNα-2a are required to keep leukemia under control in this patient.
Following our study, Dagorne et al.Citation3 reported the induction of complete hematological remission in a patient affected by AML secondary to essential thrombocytemia who received pegylated IFNα-2a at a dose of 180 µg per week. This regimen was continued for 13 mo until HSCT. The overall survival of this patient was 18 mo and death was due to HSCT-related complications.
IFNα-based immunotherapy has been shown to exert remarkable effects in patients affected by BCR/ABL-negative myeloproliferative neoplasms, especially polycythemia vera and essential thrombocythemia.Citation10 As evidenced by the cases described above, a prolonged treatment with pegylated IFNα-2a can also provide clinical benefits in individuals affected by AML secondary to myelofibrosis or essential thrombocytemia. Whether IFNα in its pegylated form exerts a therapeutic activity against AML in general remains to be determined in clinical trials. However, the data reported above coupled to the generally low toxicity of pegylated IFNα-2a provide a rationale for the use of this immunotherapeutic agent in AML patients with poor prognosis, especially in cases in which AML is secondary to myeloproliferative neoplasms and in elderly patients that are not eligible for aggressive chemotherapy.
| Abbreviations: | ||
| AML | = | acute myeloid leukemia |
| DC | = | dendritic cell |
| IFN | = | interferon |
| HSCT | = | hematopoietic stem cell transplantation |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Anguille S, Lion E, Willemen Y, Van Tendeloo VF, Berneman ZN, Smits EL. Interferon-α in acute myeloid leukemia: an old drug revisited. Leukemia 2011; 25:739 - 48; http://dx.doi.org/10.1038/leu.2010.324; PMID: 21274002
- Berneman ZN, Anguille S, Van Marck V, Schroyens WA, Van Tendeloo VF. Induction of complete remission of acute myeloid leukaemia by pegylated interferon-alpha-2a in a patient with transformed primary myelofibrosis. Br J Haematol 2010; 149:152 - 5; http://dx.doi.org/10.1111/j.1365-2141.2009.08029.x; PMID: 19995392
- Dagorne A, Douet-Guilbert N, Quintin-Roue I, Guillerm G, Couturier MA, Berthou C, et al. Pegylated interferon α2a induces complete remission of acute myeloid leukemia in a postessential thrombocythemia myelofibrosis permitting allogenic stem cell transplantation. Ann Hematol 2013; 92:407 - 9; http://dx.doi.org/10.1007/s00277-012-1560-9; PMID: 22941306
- Diamond MS, Kinder M, Matsushita H, Mashayekhi M, Dunn GP, Archambault JM, et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med 2011; 208:1989 - 2003; http://dx.doi.org/10.1084/jem.20101158; PMID: 21930769
- Anguille S, Lion E, Tel J, de Vries IJ, Couderé K, Fromm PD, et al. Interleukin-15-Induced CD56(+) Myeloid Dendritic Cells Combine Potent Tumor Antigen Presentation with Direct Tumoricidal Potential. PLoS One 2012; 7:e51851; http://dx.doi.org/10.1371/journal.pone.0051851; PMID: 23284789
- Papewalis C, Jacobs B, Wuttke M, Ullrich E, Baehring T, Fenk R, et al. IFN-alpha skews monocytes into CD56+-expressing dendritic cells with potent functional activities in vitro and in vivo. J Immunol 2008; 180:1462 - 70; PMID: 18209041
- Roothans D, Smits E, Lion E, Tel J, Anguille S. CD56 marks human dendritic cell subsets with cytotoxic potential. OncoImmunology 2013; In press http://dx.doi.org/10.4161/onci.23037
- Benjamin R, Khwaja A, Singh N, McIntosh J, Meager A, Wadhwa M, et al. Continuous delivery of human type I interferons (alpha/beta) has significant activity against acute myeloid leukemia cells in vitro and in a xenograft model. Blood 2007; 109:1244 - 7; http://dx.doi.org/10.1182/blood-2006-02-002915; PMID: 17047156
- Mesa RA, Li CY, Ketterling RP, Schroeder GS, Knudson RA, Tefferi A. Leukemic transformation in myelofibrosis with myeloid metaplasia: a single-institution experience with 91 cases. Blood 2005; 105:973 - 7; http://dx.doi.org/10.1182/blood-2004-07-2864; PMID: 15388582
- Kiladjian JJ, Chomienne C, Fenaux P. Interferon-alpha therapy in bcr-abl-negative myeloproliferative neoplasms. Leukemia 2008; 22:1990 - 8; http://dx.doi.org/10.1038/leu.2008.280; PMID: 18843285