Abstract
Regulatory T cells (Tregs) that expand in human colon cancer express retinoid-related orphan receptor γt (RORγt) and exert potent T-cell suppressive functions while mediating pro-inflammatory effects. Similar Tregs expand and drive a vicious cycle of inflammation in murine polyposis. Targeting RORγt in Tregs interrupts such a cycle and protects mice against polyposis, suggesting that a similar intervention may provide therapeutic benefits to colon cancer patients.
Keywords: :
Regulatory T Cells Are Attractive Targets for Anticancer Immunotherapy
The infiltration of colon cancer (CC) lesions by lymphocytes is an independent predictor of increased patient survival.Citation1 In addition, CC patients exhibit natural CD8+ T-cell responses that recognize and can eliminate tumor cells in an antigen-specific manner.Citation2 These observations have raised the hope that immunosurveillance mechanisms might keep neoplastic cells at bay and that vaccination strategies might efficiently eradicate CC.Citation2 Still, immunotherapeutic approaches have not generated consistent therapeutic benefits for CC patients so far. FOXP3+ regulatory T cells (Tregs) expand in cancer patients, have preferential access to neoplastic lesions and hinder antitumor CD8+ T-cell responses. Interestingly, Tregs differ significantly from effector T cells with regard to their T-cell receptor (TCR) repertoire, and therefore tend to recognize different antigens than effector T cells.Citation2 An elevated number of tumor-infiltrating Tregs has been associated with poor clinical outcomes in cohorts of patients affected by multiple types of cancer, encouraging therapeutic strategies aiming at the elimination of Tregs. There is however much controversy about the actual role of Tregs in cancer. Recent findings challenge the view that Tregs are always deleterious and rather suggest that high densities of intratumoral Tregs can correlate with better clinical outcomes in patients affected by CC and several other cancers.Citation3
The ability of Tregs to control inflammation explains their protective role in cancer. Oncogenic events and the breakdown of the epithelial barrier cause inflammation, which provides both fuel and protection to growing tumors.Citation4 In CC, a deregulated T helper 17 (TH17) response to microbial products contributes to tumor growth, and TH17 cytokines are elevated both in human CC and in murine polyposis, a setting in which the inhibition of these cytokines exert consistent protective effects.Citation5-Citation7 In humans, persistent TH17 inflammation predisposes to inflammatory bowel disease, and tumor infiltration by IL-17-expressing lymphocytes negatively correlates with patient survival in CC.Citation8 Tregs suppress TH17 cells in an interleukin (IL)-10 dependent manner.Citation9 However, when activated under inflammatory conditions, Tregs can acquire different functional properties and emerge as a prominent pro-inflammatory cell subset, hence converting into cells that they normally suppress, TH17 cells. The conversion of Tregs into TH17 cells does not seem to be highly deleterious in cancer, as it would presumably reduce the abundance of Tregs. However, cancer is marked by significant increases in both systemic and tumor-infiltrating FOXP3+ Tregs.
Different Subsets of Regulatory T Cells Can Protect or Promote Colon Cancer
Observations in support of a protective role of Tregs in CC have been obtained in mouse models. The adoptive transfer of Tregs from healthy mice protect against experimental colitis as well as against microbial-associated colitis and cancer.Citation10 Furthermore, the transfer of Tregs protects against polyposis in mice that are genetically predisposed to develop this disease.Citation7 Such a protection is generally attributed to the capacity of Tregs to suppress inflammatory reactions that are inherent to pre-neoplastic settings, including those driven by mast cells, macrophages and granulocytes, and to reduce the levels of TH17 cytokines, leading to the active regression of polyps.Citation5 However, the fact that endogenous Tregs expand and are recruited to murine polyps without any apparent detrimental effect for the latter is confounding. Obviously, there must be functional differences between Tregs isolated from healthy and polyp-ridden mice. Indeed, Tregs isolated from the latter not only do not inhibit inflammation and polyposis but also have a tendency to mediate opposite effects while remaining able to potently suppress T-cell responses.Citation5
We have recently demonstrated that pro-inflammatory Tregs can be discriminated from their counterparts as they co-express FOXP3 and retinoid-related orphan receptor γt (RORγt), which is the signature transcription factor of TH17 cells.Citation7 When we compared the effects of the ablation of TH17 cytokines with those of the ablation of RORγt, the latter was by far more protective. The significance of pro-inflammatory Tregs in the etiopathology of polyposis became evident when the Treg-specific ablation of RORγt was found to hinder inflammation and exert cytoprotective effects. The ablation of RORγt in the T-cell compartment also exacerbated T-cell responses against polyps. We concluded that the acquisition of pro-inflammatory properties by Tregs is a turning point that allows for the uncontrolled escalation of T-cell suppressive and polyposis-promoting inflammatory reactions.
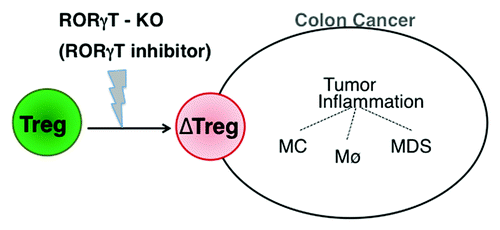
Our observations have direct implications for human cancer. We indeed detected Tregs exhibiting the phenotype and functional properties described above in the peripheral blood and tumors of CC patients. The expansion of RORγt+ Tregs in patients was strictly tumor-dependent. In line with this notion, the abundance of RORγt+ Tregs dropped upon the surgical removal of primary tumors in non-metastatic patients. There were however a few cases making exception, and in these patients RORγt+ Tregs persisted post-surgery. We are now following surgerized patients to see whether the persistence of pro-inflammatory Tregs may convey prognostic information. Based on results obtained in mouse models, we predict that the pharmacological inhibition of RORγt will provide clinical benefits to cancer patients. The administration of a novel, first generation RORγt inhibitor for 2 weeks reduced the number of polyps arising in ApcΔ468 mice, opening the path to clinical trials targeting the plasticity of Tregs in cancer patients. The same inhibitor was shown to restore the anti-inflammatory properties of Tregs isolated from CC patients. We conclude that the expression of RORγt by Tregs is causatively related to CC and that its inhibition can convey therapeutic benefits ().
Figure 1. Implication of RORγt-expressing regulatory T cells in the pathogenesis of colon cancer. Regulatory T cells (Tregs) infiltrating the tumor environment respond to cell-extrinsic cues by upregulating retinoid-related orphan receptor γt (RORγt) and hence acquiring pro-inflammatory properties. This creates a vicious cycle of inflammation that stimulates tumor progression. Strategies for the inhibition of RORγt may interrupt this cycle and exert potent antitumor effects.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006; 313:1960 - 4; http://dx.doi.org/10.1126/science.1129139; PMID: 17008531
- Khazaie K, Bonertz A, Beckhove P. Current developments with peptide-based human tumor vaccines. Curr Opin Oncol 2009; 21:524 - 30; http://dx.doi.org/10.1097/CCO.0b013e328331a78e; PMID: 19770763
- Wilke CM, Kryczek I, Wei S, Zhao E, Wu K, Wang G, et al. Th17 cells in cancer: help or hindrance?. Carcinogenesis 2011; 32:643 - 9; http://dx.doi.org/10.1093/carcin/bgr019; PMID: 21304053
- Khazaie K, Blatner NR, Khan MW, Gounari F, Gounaris E, Dennis K, et al. The significant role of mast cells in cancer. Cancer Metastasis Rev 2011; 30:45 - 60; http://dx.doi.org/10.1007/s10555-011-9286-z; PMID: 21287360
- Gounaris E, Blatner NR, Dennis K, Magnusson F, Gurish MF, Strom TB, et al. T-regulatory cells shift from a protective anti-inflammatory to a cancer-promoting proinflammatory phenotype in polyposis. Cancer Res 2009; 69:5490 - 7; http://dx.doi.org/10.1158/0008-5472.CAN-09-0304; PMID: 19570783
- Blatner NR, Bonertz A, Beckhove P, Cheon EC, Krantz SB, Strouch M, et al. In colorectal cancer mast cells contribute to systemic regulatory T-cell dysfunction. Proc Natl Acad Sci U S A 2010; 107:6430 - 5; http://dx.doi.org/10.1073/pnas.0913683107; PMID: 20308560
- Blatner NR, Mulcahy MF, Dennis KL, Scholtens D, Bentrem DJ, Phillips JD, et al. Expression of RORγt marks a pathogenic regulatory T cell subset in human colon cancer. Sci Transl Med 2012; 4:ra159; http://dx.doi.org/10.1126/scitranslmed.3004566; PMID: 23241743
- Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G, et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res 2011; 71:1263 - 71; http://dx.doi.org/10.1158/0008-5472.CAN-10-2907; PMID: 21303976
- Bos PD, Rudensky AY. Treg cells in cancer: a case of multiple personality disorder. Sci Transl Med 2012; 4:64fs44; http://dx.doi.org/10.1126/scitranslmed.3005283; PMID: 23241741
- Erdman SE, Poutahidis T. Cancer inflammation and regulatory T cells. Int J Cancer 2010; 127:768 - 79; PMID: 20518013