Abstract
Tumor-infiltrating plasmacytoid dendritic cells (pDCs) have been associated with poor patient prognosis. We have recently uncovered the ability of pDCs to activate and expand a subset of tumor-infiltrating FOXP3+ regulatory T cells that express inducible costimulator (ICOS), providing new insights into the mechanisms that govern the escape of cancer from immunosurveillance.
Regulatory T cells constitute 5–10% of peripheral CD4+ T cells and play an essential role in the active suppression of autoimmune responses, in both humans and rodents. Increasing evidence suggests that CD4+ regulatory T cells that infiltrate neoplastic lesions also play a significant role in the suppression of antitumor immunity and may represent a key mechanism by which tumors foster immune privilege. Recently, a circulating subset of thymic-derived regulatory T cells that constitutively express FOXP3 as well as inducible costimulator (ICOS) and that inhibit T-cell proliferation by an interleukin (IL)-10-dependent effect on antigen-presenting cells (APCs) has been identified in healthy subjects.Citation1 Of note, the survival, proliferation and immunosuppressive functions of these cells are strictly dependent on ICOS-mediated, rather than on CD28-mediated, co-stimulation.Citation1 Several studies have recently demonstrated that ICOS+FOXP3+ regulatory T cells abundantly infiltrate several human neoplasms, including epithelial ovarian cancer (EOC),Citation2 breast cancer,Citation3 thyroid cancerCitation4 and melanoma.Citation5 The intratumoral levels of ICOS+FOXP3+ regulatory T cells were found to predict cancer progression more robustly than the abundance of all tumor-infiltrating regulatory T cells,Citation2,Citation4 indicating that this cell subset plays a key role in tumor-elicited immunosuppression (and hence in disease progression).
Plasmacytoid dendritic cells (pDCs), a rare subset of circulating dendritic cells, have also been detected in the microenvironment of many solid tumors. Whereas pDCs are known to participate in antiviral immunity as they respond to viral infections by producing high levels of Type I interferons (IFNs), the role of these cells within the tumor microenvironment has remained unclear. Tumor-infiltrating pDCs appear to maintain a non-activated state and generally do not produce Type I IFNs, reflecting either the lack of activation stimuli in situ or the active inhibition of pDC functions by malignant cells.Citation6 Similar to high levels of regulatory T cells, abundant tumor infiltration by pDCs has also been associated with poor clinical outcomes,Citation2,Citation7 suggesting a potential link between pDCs and regulatory T cell-mediated immunosuppression. Indeed, a role for pDCs in the activation and expansion of regulatory T cells had been previously demonstrated in models of asthma, transplantation and oral tolerance.
But how do pDCs drive regulatory T cell-mediated immunosuppression in cancer? Tumor pDCs express high levels of the ICOS ligand (ICOS-L), along with low levels of CD80 and CD86,Citation8 a unique constellation of co-stimulatory molecules that allows for the optimal stimulation of ICOS+ regulatory T cells.Citation1 In fact, pDCs but not other APCs were found to stimulate the activation and expansion of ICOS+ FOXP3+ regulatory T cells by providing ICOS co-stimulation.Citation2 Within tumor lesions, pDCs are located in the close proximity of ICOS+FOXP3+ regulatory T cells, and their abundance directly correlates with that of this regulatory T-cell subset.Citation2 Furthermore, independent studies have demonstrated that both pDCs and ICOS+ regulatory T cells constitute strong predictors of disease progression and poor clinical outcome in patients affected by ovarian,Citation2 breastCitation3 and thyroid cancer.Citation4 Thus, pDCs promote immunosuppression by activating and expanding ICOS+FOXP3+ T regulatory cells through ICOS co-stimulation (). This signaling pathway appears to be important for tumor progression as it directly influences the prognosis of patients bearing metastatic cancers.
Figure 1. Role of plasmacytoid dendritic cells and ICOS+ regulatory T cells in tumor immunosuppression. Bone marrow-derived plasmacytoid dendritic cells (pDCs) and thymic-derived ICOS+FOXP3+ regulatory T cells are recruited from the circulation into the tumor microenvironment. Tumor-infiltrating pDCs express high levels of the ICOS ligand (ICOSL), which co-stimulates ICOS+FOXP3+ regulatory T cells in the context of tumor-associated antigen presentation by pDCs or bystander antigen-presenting cells (APCs). This drives the activation and proliferation of ICOS+FOXP3+ regulatory T cells, leading to a preferential accumulation of this regulatory T-cell subset within the tumor microenvironment. Upon re-encounter with the tumor-associated antigen, ICOS+FOXP3+ regulatory T cells secrete interleukin (IL)-10, hence suppressing the effector functions of tumor-specific CD4+ and CD8+ T cells. Thus, the infiltration of neoplastic lesions by pDCs favors the establishment of an immunosuppressive microenvironment via the activation and expansion of ICOS+FOXP3+ regulatory T cells, de facto favoring disease progression.
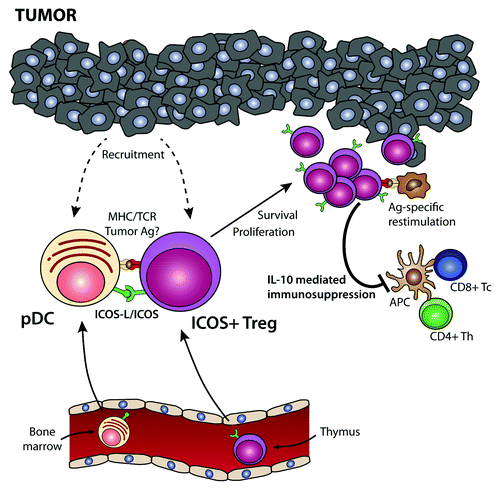
Although the ability of pDCs to stimulate ICOS+FOXP3+ regulatory T cells requires MHC-T-cell receptor (TCR) interactions, the nature of the antigen presented by pDCs is currently unknown. It is generally accepted that pDCs have a limited capacity to internalize exogenous antigens by phagocytosis and that they may rather be specialized in presenting endogenous molecules. However, tumor-infiltrating pDCs were found to induce a strong expansion of ICOS+FOXP3+ regulatory T cells in cancer patients, raising the possibility that they also present tumor-associated antigens (). Interestingly, a recent study has shown that pDCs are indeed able to take up exogenous tumor-derived antigens via specific receptors.
Taken together, these findings provide novel insights into the factors that mediate cancer-elicited immunosuppression and identify new molecular targets for therapeutic interventions. In this setting, one therapeutic strategy would be to block ICOS-L/ICOS co-stimulation, to reduce the expansion and functions of ICOS+ regulatory T cells within the tumor microenvironment. Alternatively, the recruitment of pDCs into neoplastic lesions could be specifically targeted. EOC cells have been shown to produce large amounts of the CXCR4 ligand CXCL12 (also known as stromal cell-derived factor1, SDF1), which directly recruits pDCs into the tumor microenvironment.Citation6 In line with our model, inhibitors of CXCL12-CXCR4 signaling axis have previously been shown to reduce tumor growth in mouse models of EOC.Citation9,Citation10 Finally, Toll-like receptor (TLR)7 or TLR9 ligands could be used to promote robust antitumor immune responses by virtue of their capacity of activating pDCs to produce type I IFNs and of differentiating pDCs into cytotoxic effector cells.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Ito T, Hanabuchi S, Wang YH, Park WR, Arima K, Bover L, et al. Two functional subsets of FOXP3+ regulatory T cells in human thymus and periphery. Immunity 2008; 28:870 - 80; http://dx.doi.org/10.1016/j.immuni.2008.03.018; PMID: 18513999
- Conrad C, Gregorio J, Wang YH, Ito T, Meller S, Hanabuchi S, et al. Plasmacytoid dendritic cells promote immunosuppression in ovarian cancer via ICOS costimulation of Foxp3(+) T-regulatory cells. Cancer Res 2012; 72:5240 - 9; http://dx.doi.org/10.1158/0008-5472.CAN-12-2271; PMID: 22850422
- Faget J, Bendriss-Vermare N, Gobert M, Durand I, Olive D, Biota C, et al. ICOS-ligand expression on plasmacytoid dendritic cells supports breast cancer progression by promoting the accumulation of immunosuppressive CD4+ T cells. Cancer Res 2012; 72:6130 - 41; http://dx.doi.org/10.1158/0008-5472.CAN-12-2409; PMID: 23026134
- Yu H, Huang X, Liu X, Jin H, Zhang G, Zhang Q, et al. Regulatory T cells and plasmacytoid dendritic cells contribute to the immune escape of papillary thyroid cancer coexisting with multinodular non-toxic goiter. Endocrine 2012; In press http://dx.doi.org/10.1007/s12020-012-9853-2; PMID: 23264145
- Martin-Orozco N, Li Y, Wang Y, Liu S, Hwu P, Liu YJ, et al. Melanoma cells express ICOS ligand to promote the activation and expansion of T-regulatory cells. Cancer Res 2010; 70:9581 - 90; http://dx.doi.org/10.1158/0008-5472.CAN-10-1379; PMID: 21098714
- Zou W, Machelon V, Coulomb-L’Hermin A, Borvak J, Nome F, Isaeva T, et al. Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nat Med 2001; 7:1339 - 46; http://dx.doi.org/10.1038/nm1201-1339; PMID: 11726975
- Treilleux I, Blay JY, Bendriss-Vermare N, Ray-Coquard I, Bachelot T, Guastalla JP, et al. Dendritic cell infiltration and prognosis of early stage breast cancer. Clin Cancer Res 2004; 10:7466 - 74; http://dx.doi.org/10.1158/1078-0432.CCR-04-0684; PMID: 15569976
- Ito T, Yang M, Wang YH, Lande R, Gregorio J, Perng OA, et al. Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand. J Exp Med 2007; 204:105 - 15; http://dx.doi.org/10.1084/jem.20061660; PMID: 17200410
- Kajiyama H, Shibata K, Terauchi M, Ino K, Nawa A, Kikkawa F. Involvement of SDF-1alpha/CXCR4 axis in the enhanced peritoneal metastasis of epithelial ovarian carcinoma. Int J Cancer 2008; 122:91 - 9; http://dx.doi.org/10.1002/ijc.23083; PMID: 17893878
- Ray P, Lewin SA, Mihalko LA, Schmidt BT, Luker KE, Luker GD. Noninvasive imaging reveals inhibition of ovarian cancer by targeting CXCL12-CXCR4. Neoplasia 2011; 13:1152 - 61; PMID: 22241961