Abstract
Human γδ T cells possess broad antitumor reactivity and are involved in the control of viral infections. We have recently described multifunctional γδ T cells induced by cytomegalovirus after allogenic stem cell transplantation, placing γδ T cells and their receptors in the spotlight for the development of novel anticancer immunotherapies.
Over the last few decades, cytomegalovirus (CMV) reactivation was considered a major life-threatening complication of allogenic stem cell transplantation (allo-SCT). Nowadays, a sensitive monitoring for early CMV reactivation combined with the availability of effective antiviral treatments has rendered the CMV-related death of transplanted patients a rare event. Fortuitously, such an improved control over CMV reactivation has facilitated observational studies in large cohorts of transplanted patients, highlighting a surprising beneficial association between CMV reactivation and a reduced risk of leukemic relapse.Citation1 So far, however, how viral reactivation would provide a protection from leukemic relapse has remained unclear. Among various possibilities, it has been proposed that natural killer (NK) cells may cross-react with CMV-infected cells and tumor cells by responding to CMV-infected residual AML blasts.Citation1 In a recent issue of Leukemia, we propose an additional and perhaps even more physiologically relevant explanation for this apparent paradox, i.e., that γδ T cells play a pivotal role in the CMV-induced clearance of residual tumor cells.Citation2 We observed that these unconventional T cells not only expand in patients that reactivate CMV upon allo-SCT but also react against both CMV-infected and leukemic cells. Thus, we propose that multifunctional γδ T cells could substantially contribute to the protection from leukemic relapse that is associated with CMV reactivation after allo-SCT.
In humans, circulating γδ T cells are a minor population that mostly expresses T-cell receptors (TCRs) containing the Vδ2 and Vγ9 gene segments (so-called Vδ2pos γδ T cells).Citation3 In contrast, epithelial γδ T cells mainly express TCRs composed of Vδ1 or Vδ3 chains (Vδ2neg γδ T cells) optionally in combination with CD8αα. Over the last decade, many studies have implicated Vδ2neg γδ T cells in the anti-CMV response as well as in antitumor immunosurveillance, but the first report on the cross-reactivity of these cells against CMV and cancer originated from the isolation of Vδ2neg γδ T-cell clones from kidney transplant recipients.Citation4 At least in some of these clones, the double reactivity was mediated by a γδ TCR recognizing a common stress antigen that was upregulated on both CMV-infected and transformed intestinal epithelial cells,Citation5 explaining why CMV infection alone could induce an immune cell population reacting against both CMV and cancer. Conversely, in our study, gene transfer experiments using γδ TCRs isolated from cross-reactive Vδ2neg γδ T-cell clones demonstrated the crucial involvement of the γδ TCR in tumor reactivity but not in the recognition of CMV-infected cells, suggesting that—at least in these Vδ2neg γδ T-cell clones—anti-CMV reactivity relied on receptors other than the γδ TCR. This brings up a major issue in the γδ T-cell research field, that is, the poor understanding of the mechanisms of γδ T-cell activation as well of the antigens recognized by γδ TCRs. In this respect, one important finding of our study is the identification of CD8αα as a co-stimulatory molecule for the activation of defined γδ TCRs. The expression of CD8αα on γδ T cells has previously been described, yet so far there were no reports on its function. In αβ T cells, the CD8αβ heterodimer serves as co-receptor for the αβ TCR, restricting its interaction to antigens presented on MHC class I molecules. Conversely, γδ TCRs recognize antigens independently of MHC molecules, suggesting that the co-activating function of CD8αα is likely to rely on alternative mechanisms. The precise mechanisms whereby CD8αα delivers co-stimulatory signals in this setting remain to be elucidated. Nevertheless, we observed a striking increase of circulating CD8αα+ γδ T cells in CMV-reactivating individuals in our patient cohort as well as in an additional independent cohort of congenitally CMV-infected neonates, implying that CD8αα expression by γδ T cells represents a pathophysiologically relevant phenomenon in vivo.
The demonstration that a proportion of γδ T cells can cross-recognize CMV and a broad panel of hematological cancer cells make them particularly attractive for clinical applications, such as adoptive cell transfer-based immunotherapy. In the context of allo-SCT, a situation favoring the reactivity of re-infused cells against CMV and leukemia in the absence of graft vs. host disease (GVHD) might be achieved with stem cell grafts enriched for γδ T cells (). To this end, we and others are nowadays conducting clinical trials using stem cell grafts depleted of αβ T cells and B cells (NTR2463 and NTR3079).Citation6 Intriguingly, αβ TCR/CD19-depleted, but usually not CD3/CD19-depleted, grafts reconstitute very rapidly a broad αβ T-cell repertoire (J Kuball, unpublished observations), suggesting a very broad immunoregulatory role for γδ T cells that has recently also been proposed by others.Citation7 As an alternative, umbilical cord blood grafts can be used as a third party source of stem cells. These grafts typically contain high percentages of γδ T cells, and we have demonstrated that CMV- and leukemia-reactive γδ T cells can also be obtained from such an antigen-naïve repertoire. Importantly, all good manufacturing practice-grade clinical tools for the preparation of enriched stem cells grafts are available. Finally, as our results suggest a central role for CD8αα+ γδ T cells in anti-CMV immune responses, the isolation of these cells could be envisioned, although their precise function would have first to be deeply investigated.
Figure 1. Antitumor strategies based on γδ T cells. (A) The cell preparation for an “innate allogenic stem cell transplantation” (allo-SCT) from conventional or third party donors may selectively contain or be enriched for γδ T cells to provide anti-cytomegalovirus (CMV) and antitumor protection in the absence of graft versus host disease (GVHD). (B) In a complementary “autologous engineered transplantation,” T cells are isolated from cancer patients, expanded and engineered to express γδ TCRs (optimized by combinatorial-γδTCR-chain exchange) ex vivo. Reprogrammed T cells are subsequently re-infused into the patient, where they specifically recognize and kill tumor cells.
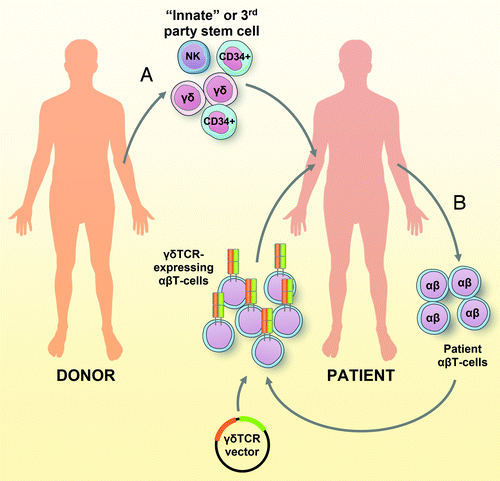
Complementary to this “innate allo-SCT” approach, γδ TCRs with broad tumor-reactivity could be characterized and used to reprogram patient-derived conventional αβ T cellsCitation8 (). Given the non-MHC-restricted antigen recognition pattern of γδ TCRs, defined γδ TCRs could—in contrast to αβ TCRs—be applied to a broad patient population in the absence of matched HLA types. Also, exogenous γδ TCR chains do not pair with their endogenous αβ counterparts, preventing the creation of novel TCRs with unpredictable (auto-) reactivity. As we have previously shown, introducing defined γδ TCRs effectively reprograms αβ T cells to kill a broad collection of tumor cells in vitro and in vivo.Citation9 In this setting, we also established a technique called combinatorial-γδTCR-chain exchange (CTE), allowing for the design of γδ TCRs with enhanced functional avidity toward malignant but not healthy tissues.Citation10 By exploiting the abundance, potent cytotoxic machinery and proliferative competence of αβ T cells even in advanced stages of disease, the engineering of autologous immune cells with such receptors would allow for the generation of large numbers of tumor-reactive T cells while tackling the major limitations of current approaches based on engineered αβ TCRs. Thus, γδ T cells and their receptors stand out as a promising avenue toward the development of new antitumor immunotherapies.
| Abbreviations: | ||
| allo-SCT | = | allogeneic stem cell transplantation |
| CMV | = | cytomegalovirus |
| CTE | = | combinatorial γδ TCR engineering |
| GVHD | = | graft versus host disease |
| TCR | = | T-cell receptor |
Disclosure of Potential Conflicts of Interest
Clinical trials were supported by Miltenyi Biotec. This work was supported by grants of the ZonMW 43400003, VIDI-ZonMW 917.11.337, LSBR 0902, AICR 10-736, KWF UU-2010-4669.
References
- Elmaagacli AH, Steckel NK, Koldehoff M, Hegerfeldt Y, Trenschel R, Ditschkowski M, et al. Early human cytomegalovirus replication after transplantation is associated with a decreased relapse risk: evidence for a putative virus-versus-leukemia effect in acute myeloid leukemia patients. Blood 2011; 118:1402 - 12; http://dx.doi.org/10.1182/blood-2010-08-304121; PMID: 21540462
- Scheper W, van Dorp S, Kersting S, Pietersma F, Lindemans C, Hol S, et al. γδT cells elicited by CMV reactivation after allo-SCT cross-recognize CMV and leukemia. Leukemia 2013; In press http://dx.doi.org/10.1038/leu.2012.374; PMID: 23277330
- Bonneville M, O’Brien RL, Born WK. Gammadelta T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol 2010; 10:467 - 78; http://dx.doi.org/10.1038/nri2781; PMID: 20539306
- Halary F, Pitard V, Dlubek D, Krzysiek R, de la Salle H, Merville P, et al. Shared reactivity of V{delta}2(neg) {gamma}{delta} T cells against cytomegalovirus-infected cells and tumor intestinal epithelial cells. J Exp Med 2005; 201:1567 - 78; http://dx.doi.org/10.1084/jem.20041851; PMID: 15897274
- Willcox CR, Pitard V, Netzer S, Couzi L, Salim M, Silberzahn T, et al. Cytomegalovirus and tumor stress surveillance by binding of a human γδ T cell antigen receptor to endothelial protein C receptor. Nat Immunol 2012; 13:872 - 9; http://dx.doi.org/10.1038/ni.2394; PMID: 22885985
- Oevermann L, Lang P, Feuchtinger T, Schumm M, Teltschik HM, Schlegel P, et al. Immune reconstitution and strategies for rebuilding the immune system after haploidentical stem cell transplantation. Ann N Y Acad Sci 2012; 1266:161 - 70; http://dx.doi.org/10.1111/j.1749-6632.2012.06606.x; PMID: 22901267
- Vantourout P, Hayday A. Six-of-the-best: unique contributions of γδ T cells to immunology. Nat Rev Immunol 2013; 13:88 - 100; http://dx.doi.org/10.1038/nri3384; PMID: 23348415
- Marcu-Malina V, van Dorp S, Kuball J. Re-targeting T-cells against cancer by gene-transfer of tumor-reactive receptors. Expert Opin Biol Ther 2009; 9:579 - 91; http://dx.doi.org/10.1517/14712590902887018; PMID: 19368527
- Marcu-Malina V, Heijhuurs S, van Buuren M, Hartkamp L, Strand S, Sebestyen Z, et al. Redirecting αβ T cells against cancer cells by transfer of a broadly tumor-reactive γδT-cell receptor. Blood 2011; 118:50 - 9; http://dx.doi.org/10.1182/blood-2010-12-325993; PMID: 21566093
- Gründer C, van Dorp S, Hol S, Drent E, Straetemans T, Heijhuurs S, et al. γ9 and δ2CDR3 domains regulate functional avidity of T cells harboring γ9δ2TCRs. Blood 2012; 120:5153 - 62; http://dx.doi.org/10.1182/blood-2012-05-432427; PMID: 23018643