Abstract
Breast tumors infiltrated by matrix metalloprotease 11 (MMP-11)+ mononuclear inflammatory cells are prone to form metastases; express high levels of interleukin (IL)-1, IL-5, IL-6, IL-17, interferon β (IFNβ) and NFκB; and exhibit an increased CD68+/(CD3+CD20+) cell ratio at their invasive front. These factors, which are implicated in the crosstalk between tumors and their inflammatory microenvironment, may emerge as attractive prognostic factors and therapeutic targets.
The development of an invasive cancer is not only the result of genetic changes in neoplastic cells but also stems from a complex interplay between neoplastic cells and their stroma. Immune cells constitute the most prominent cellular component of the tumor microenvironment. Initially, neoplastic and stromal cells respond to hypoxia and necrosis (caused by the unrestrained tumor cell proliferation), by releasing a number of growth factors and cytokines that recruit monocytes and macrophages. Historically, tumor-infiltrating leukocytes have been considered as an endogenous defense mechanism against developing tumors. However, accumulating evidence indicates that diverse immune cells exert either antitumor effects (representing an aborted attempt of the immune system to reject neoplasms) or pro-tumor functions, as they secrete cytokines, growth factors, chemokines and proteases that stimulate angiogenesis as well as the proliferation, migration and invasive potential of cancer cells.Citation1 All these aspects of the crosstalk between malignant and stromal cells impact on therapeutic responses.Citation2
In order to better characterize the mononuclear inflammatory cells (MICs) that infiltrate breast cancer lesions and their impact on distant metastasis, we investigated the expression of matrix metalloproteases (MMPs) and tissue inhibitors of metalloproteases (TIMPs) by these stromal cells. MMPs play an essential role in the degradation of stromal connective tissue and basement membrane components, which are key elements in tumor invasion and metastasis. In addition, MMPs cleave pro-apoptotic factors hence promoting apoptosis resistance in tumor cells, and regulate cancer-related angiogenesis.Citation3 The activity of MMPs is specifically inhibited by TIMPs, a heterogeneous group of multifunctional proteins that are also involved in the regulation of cell proliferation and apoptosis. We found that 32% of breast carcinomas analyzed were infiltrated by MICs exhibiting a high MMP/TIMP expression profile, which was associated with a high rate of distant metastasis (97.6%). Conversely, patients whose tumor-infiltrating MICs exhibited a low MMP/TIMP expression profile has a comparatively lower propensity for metastatic disease (26.9%). Such metastasis-related MICs were specifically characterized by the overexpression of MMP-7, MMP-9, MMP-11, MMP-13, MMP-14, TIMP-1 and TIMP-2.Citation4 MMP-11 (also known as stromelysin 3) was the most frequently expressed factor of all these (as it was found in 85.7% of MICs exhibiting a high MMP/TIMP profile expression but only in 4.6% of MICs exhibiting a low MMP/TIMP ratio). Hence, the expression of MMP-11 may constitute a useful biological marker for pro-metastatic MICs.
As a follow-up of this study, we recently investigated the relationship between the development of distant metastases and 65 intratumoral factors associated with inflammation and tumor progression in a population of cancer patients prognostically stratified in two groups according to MMP-11 expression by intratumoral MICs.Citation5 Among the 65 factors analyzed, only A disintegrin and metalloproteinase (ADAM)-8, ADAM-10, ADAM-15, ADAM-23, ADAM with thrombospondin motifs (ADAMTS)-1, ADAMTS-2, ADAMTS-15, CCL-3, interleukin (IL)-1, IL-5, IL-6, IL-8, IL-17, IL-18, interferon β (IFNβ) IL-1 receptor-associated kinase 4 (IRAK-4), MMP-1, myeloid differentiation 88 (MYD88) and NFκB were linked to MMP-11 expression by MICs, as demonstrated by real-time PCR, with IL-1, IL-5, IL-6, IL-17, IFNβ and NFκB being those most differentially expressed in the two group of patients. Thereafter, we confirmed in a wider patient population that these factors are indeed expressed to high levels in tumors infiltrated by MMP-11+ MICs. Of note, all these proteins are of great biological interest because of their strict relationship with tumor progression. For instance, IL-1 is essentially produced by activated macrophages and upregulates the expression of a great variety of proteins including IL-5, IL-6, IFNβ, collagenases and several oncoproteins (e.g., FOS, MYC, JUN). NFκB regulates the expression of several anti-apoptotic proteins such as BCL-XL, BCL-2, X-linked inhibitor of apoptosis (XIAP), caspase-8 and FADD-like apoptosis regulator (CFLAR), baculoviral IAP repeat containing (BIRC)2, BIRC3 and survivin, as well as of factors associated with tumor progression, such as cyclin D1, MYC and cyclooxygenase-2 (COX-2).Citation2 Moreover, our finding that IL-17 expression decrease by at least 100-fold in tumors that do not develop metastasis as compared with tumors that do so is particularly interesting. In the last few years, indeed, IL-17 has been considered as a key link between adaptive and innate immunity and also plays a critical role in inflammation and autoimmune diseases. Of note, TH17 cytokines including IL-17 itself regulate the expression of several fibroblast-derived factorsCitation6 that also contribute to inflammatory processes and cancer progression.Citation7,Citation8
More recently, we have investigated the clinical relevance of the relative amount of inflammatory cells, including (CD68+) macrophages, (CD3+) T cells and (CD20+) B cells, at the invasive of breast carcinomas,Citation9 considering that this is where some of the most important interactions between cancer cells and the tumor stroma take place. We observed that a high CD68+/(CD3+CD20+) cell ratio is directly associated with a higher probability of distant metastases as well as with a MMP-11+ MIC phenotype.
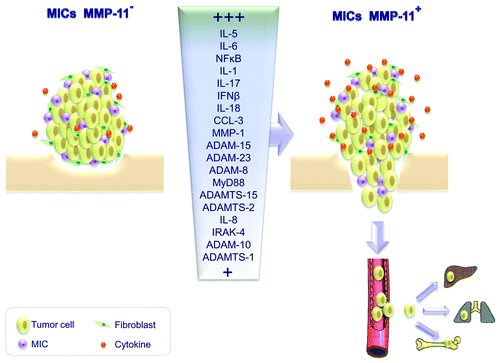
Globally, our results suggest that MMP-11 expression by MICs coupled with the expression of pro-inflammatory proteins support tumor escape and invasion, hence promoting metastasis (). These considerations led us to believe that the phenotype of stromal immune cells, in particular MMP-11 expression levels, should be included into traditional classification schemes to provide new prognostic and predictive tools to clinicians. In addition, some of the immune cells and molecules that are implicated in the crosstalk between the tumor and its inflammatory microenvironment may emerge as attractive targets for the development of novel therapies against breast cancer.
Figure 1. Effects of matrix metalloprotease 11 (MMP-11) expression by mononuclear inflammatory cells on the cytokine profile and metastatic potential of breast cancer. Tumors infiltrated by mononuclear inflammatory cells (MICs) that do not express MMP-11 release less pro-inflammatory factors than tumors containing MMP-11+ MICs. These latter cells mainly express interleukin (IL)-1, IL-5, IL-6, IL-17, interferon β (IFNβ) and NFκB and are associated with poor prognosis. MMP-11 expression by MICs coupled to the upregulation of pro-inflammatory proteins appears indeed to promote the metastatic spread of breast carcinoma cells.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 2012; 21:309 - 22; http://dx.doi.org/10.1016/j.ccr.2012.02.022; PMID: 22439926
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010; 140:883 - 99; http://dx.doi.org/10.1016/j.cell.2010.01.025; PMID: 20303878
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2002; 2:161 - 74; http://dx.doi.org/10.1038/nrc745; PMID: 11990853
- González LO, Pidal I, Junquera S, Corte MD, Vázquez J, Rodríguez JC, et al. Overexpression of matrix metalloproteinases and their inhibitors in mononuclear inflammatory cells in breast cancer correlates with metastasis-relapse. Br J Cancer 2007; 97:957 - 63; PMID: 17848954
- Eiró N, González L, González LO, Fernandez-Garcia B, Lamelas ML, Marín L, et al. Relationship between the inflammatory molecular profile of breast carcinomas and distant metastasis development. PLoS One 2012; 7:e49047; http://dx.doi.org/10.1371/journal.pone.0049047; PMID: 23145063
- Qiu Z, Dillen C, Hu J, Verbeke H, Struyf S, Van Damme J, et al. Interleukin-17 regulates chemokine and gelatinase B expression in fibroblasts to recruit both neutrophils and monocytes. Immunobiology 2009; 214:835 - 42; http://dx.doi.org/10.1016/j.imbio.2009.06.007; PMID: 19628296
- Liao D, Luo Y, Markowitz D, Xiang R, Reisfeld RA. Cancer associated fibroblasts promote tumor growth and metastasis by modulating the tumor immune microenvironment in a 4T1 murine breast cancer model. PLoS One 2009; 4:e7965; http://dx.doi.org/10.1371/journal.pone.0007965; PMID: 19956757
- Del Casar JM, González LO, Alvarez E, Junquera S, Marín L, González L, et al. Comparative analysis and clinical value of the expression of metalloproteases and their inhibitors by intratumor stromal fibroblasts and those at the invasive front of breast carcinomas. Breast Cancer Res Treat 2009; 116:39 - 52; http://dx.doi.org/10.1007/s10549-009-0351-z; PMID: 19241156
- Eiró N, Pidal I, Fernandez-Garcia B, Junquera S, Lamelas ML, del Casar JM, et al. Impact of CD68/(CD3+CD20) ratio at the invasive front of primary tumors on distant metastasis development in breast cancer. PLoS One 2012; 7:e52796; http://dx.doi.org/10.1371/journal.pone.0052796; PMID: 23300781