Abstract
Novel therapeutic strategies are needed to counteract breast cancer-associated immunosuppression. Silencing the expression of galectin-1 in a breast carcinoma model inhibited tumor growth and prevented lung metastasis by reducing the frequency and immunosuppressive activity of CD4+CD25+ FOXP3+ regulatory T cells.
Breast carcinoma is the primary cause of cancer-related death in women. Mortality mainly ensues the metastatic spread of tumor cells to other organs, which involves the acquisition of invasive features by malignant cells as well as their ability to elude antitumor immune responses.Citation1 Breast tumors employ diverse strategies to thwart the attacks of the immune system and create a tolerogenic microenvironment, including the production of immunosuppressive cytokines such as transforming growth factor β (TGFβ) and interleukin (IL)-10Citation2 as well as the expression of pro-apoptotic molecules including FAS ligandCitation3 and TRAIL,Citation4 which may act in concert to dampen the activity of effector T cells. In addition, accumulating evidence highlights the importance of regulatory cell populations,Citation5 including myeloid-derived suppressor cells (MDSCs), regulatory T cells (Tregs), tolerogenic (plasmacytoid or myeloid) dendritic cells (DCs) and the recently-discovered adipose-derived stem cells (ASCs).Citation6 These immunosuppressive mechanisms can either be pre-existing, arise through the outgrowth of escape tumor variants, or be established in the course of immunoediting, namely, the process whereby the immune system “sculpts” the tumor by eliminating some, but not all, malignant cells.Citation1 It has been proposed that the blockade of these immunosuppressive mechanisms might be effective in combination with other immunotherapeutic strategies to overcome immunological tolerance, promote tumor regression and prevent metastatic disease.Citation1 Thus, a clear understanding of the strategies devised by tumors to counteract immune responses may contribute to the design of novel, rational immunotherapeutic strategies.
Research over the past decade has identified key roles for lectin-glycan interactions in cancer immunoediting and metastasis.Citation7 Galectins are endogenous lectins characterized by their ability to recognize multiple N-acetyllactosamine [Galβ1–4NAcGlc] sequences, which may be displayed on both N- and O-glycans of cell surface glycoconjugates.Citation7 Galectin-1 (Gal-1), a prototypical member of this family, has emerged as a key regulator of immune cell homeostasis owing to its ability to shape both the T-cell and DC compartments.Citation7 Interestingly, Gal-1 is abundantly expressed at sites of tumor growth and influences disease progression by promoting angiogenesis and favoring immune escape.Citation7,Citation8 However, in spite of considerable progress, the contribution of the Gal-1-glycan axis to breast cancer-associated immunosuppression and its link to metastasis have not yet been carefully examined.
In a recent issue of Cancer Research,Citation9 we demonstrated that Gal-1 is highly expressed by human and murine breast carcinoma and significantly contributes to immunosuppression during the metastatic progression of the disease. The analysis of 55 human breast cancer biopsies revealed a positive correlation between Gal-1 expression and the Scarff-Bloom-Richardson clinical grade, suggesting that this lectin is upregulated during mammary carcinogenesis. Using RNA interference strategies, we specifically depleted Gal-1 in highly metastatic 4T1 breast tumor cells, generating Gal-1-deficient tumors. As a consequence of Gal-1 silencing, tumor growth and lung metastasis were substantially reduced. Moreover, a careful analysis of tumor-associated CD4+ T cells revealed a lower frequency and diminished immunosuppressive activity of CD4+CD25+FOXP3+ Tregs in the spleen, tumor-draining lymph nodes, primary malignant lesions and metastatic lungs of mice bearing Gal-1-depleted tumors, as compared with animals bearing wild-type 4T1 cells. Silencing Gal-1 in tumor cells also blunted the synthesis of TH2 cytokines by the host immune system, as shown in the spleen or tumor-draining lymph nodes from mice bearing 4T1-depleted tumors, which produced considerably lower amounts of IL-5 and IL-10, while exhibiting a lower IL-10 to interferon γ (IFNγ) ratio, than to their wild-type counterparts.
The role of breast cancer-derived Gal-1 in systemic immunosuppression was further demonstrated in vivo in a series of tumor transplantation experiments. When mice were simultaneously challenged with a wild-type (Gal-1-sufficient) and Gal-1-deficient tumor, the presence of the former was sufficient to imprint a dominant systemic immunosuppressive effect that compromised the development of an effective antitumor response against 4T1 cancer cells lacking Gal-1. Moreover, the depletion of Gal-1 in 4T1 cells restored the ability of BALB/c mice to reject an allogenic B16 melanoma injected in the contralateral flank, suggesting that targeting Gal-1 unleashes otherwise repressed systemic T cell responses in vivo.
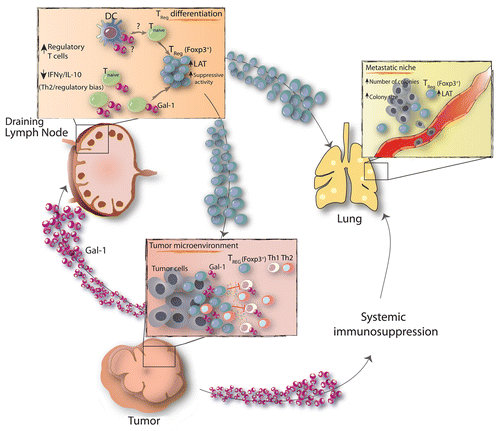
How do mice bearing Gal-1-deficient tumors mount such effective T-cell responses? We found that Tregs isolated from mice bearing Gal-1-depleted tumors exert poor immunosuppressive activity when co-cultured with conventional T cells. Consistent with these findings, we observed a considerable downregulation of linker of activated T cells (LAT) in Tregs isolated from the metastatic lungs or from the tumor-draining lymph nodes of mice bearing Gal-1-deficient tumors. This result is particularly relevant in view of the recent association between LAT expression levels and the immunosuppressive activity of Tregs.Citation10 Collectively, our results demonstrate that Gal-1 expression by the primary tumor facilitates the development of distant lung metastasis through the induction of local and systemic immunosuppression, in particular via the modulation of FOXP3+ Tregs (). This study provides proof-of-principle for targeting Gal-1 in the tumor microenvironment to counteract breast cancer-associated immunosuppression, restrain tumor growth and prevent metastatic disease.
Figure 1. Galectin-1 contributes to the tumorigenic and metastatic potential of 4T1 breast tumors by promoting local and systemic immunosuppression. Tumor-derived galectin-1 (Gal-1) promotes the differentiation of highly suppressive FOXP3+LAT+ regulatory T cells (Tregs), which migrate from tumor-draining lymph nodes to the lung and facilitate the establishment of metastatic foci. The expression of Gal-1 by breast cancer cells favors metastatic seeding by profoundly influencing the frequency and immunosuppressive capacity of Tregs.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 2011; 331:1565 - 70; http://dx.doi.org/10.1126/science.1203486; PMID: 21436444
- Heckel MC, Wolfson A, Slachta CA, Schwarting R, Salgame P, Katsetos CD, et al. Human breast tumor cells express IL-10 and IL-12p40 transcripts and proteins, but do not produce IL-12p70. Cell Immunol 2011; 266:143 - 53; http://dx.doi.org/10.1016/j.cellimm.2010.09.010; PMID: 21055733
- Zhang B, Sun T, Xue L, Han X, Zhang B, Lu N, et al. Functional polymorphisms in FAS and FASL contribute to increased apoptosis of tumor infiltration lymphocytes and risk of breast cancer. Carcinogenesis 2007; 28:1067 - 73; http://dx.doi.org/10.1093/carcin/bgl250; PMID: 17183065
- Bilski A, Pasz-Walczak G, Kubiak R, Sek P, Chalubinska J, Fendler W, et al. TRAIL protein expression in breast cancer cells correlates with nuclear grade. Arch Med Sci 2010; 6:545 - 51; http://dx.doi.org/10.5114/aoms.2010.14466; PMID: 22371798
- Coussens LM, Pollard JW. Leukocytes in mammary development and cancer. Cold Spring Harb Perspect Biol 2011; 3:3; http://dx.doi.org/10.1101/cshperspect.a003285; PMID: 21123394
- Razmkhah M, Jaberipour M, Erfani N, Habibagahi M, Talei AR, Ghaderi A. Adipose derived stem cells (ASCs) isolated from breast cancer tissue express IL-4, IL-10 and TGF-β1 and upregulate expression of regulatory molecules on T cells: do they protect breast cancer cells from the immune response?. Cell Immunol 2011; 266:116 - 22; http://dx.doi.org/10.1016/j.cellimm.2010.09.005; PMID: 20970781
- Rabinovich GA, Croci DO. Regulatory circuits mediated by lectin-glycan interactions in autoimmunity and cancer. Immunity 2012; 36:322 - 35; http://dx.doi.org/10.1016/j.immuni.2012.03.004; PMID: 22444630
- Croci DO, Salatino M, Rubinstein N, Cerliani JP, Cavallin LE, Leung HJ, et al. Disrupting galectin-1 interactions with N-glycans suppresses hypoxia-driven angiogenesis and tumorigenesis in Kaposi’s sarcoma. J Exp Med 2012; 209:1985 - 2000; http://dx.doi.org/10.1084/jem.20111665; PMID: 23027923
- Dalotto-Moreno T, Croci DO, Cerliani JP, Martinez-Allo VC, Dergan-Dylon S, Méndez-Huergo SP, et al. Targeting galectin-1 overcomes breast cancer-associated immunosuppression and prevents metastatic disease. Cancer Res 2013; 73:1107 - 17; http://dx.doi.org/10.1158/0008-5472.CAN-12-2418; PMID: 23204230
- Chuck MI, Zhu M, Shen S, Zhang W. The role of the LAT-PLC-gamma1 interaction in T regulatory cell function. J Immunol 2010; 184:2476 - 86; http://dx.doi.org/10.4049/jimmunol.0902876; PMID: 20130215