Abstract
The reciprocal interaction between cancer stem cells and their niche is important for oncogenesis and tumor progression, but the factors involved in this interrelationship remain largely unknown. We have recently demonstrated that osteopontin plays a crucial role in the crosstalk between CD44+ colorectal cancer stem cells and tumor-associated macrophages.
In normal tissues, stem cells reside in a specialized niche that regulates their properties and fates through cell-to-cell contacts and soluble factors including several cytokines. The components of this niche thus provide context signals to maintain stem cells in an equilibrium between self-renewal and differentiation. Tumors are generally viewed as abnormal tissues, and only a small fraction of malignant cells are considered as cancer stem cells (CSCs), owing to their superior tumor-initiating capacity. Emerging evidence shows that, similar to their normal counterparts, CSCs also reside in a specific microenvironment that is referred to as CSC niche. Thus, similar to normal stem cells, CSCs are regulated by, and in turn influence, stromal cells within the tumor microenvironment. These stromal cell compartments, including endothelial cells, infiltrating leukocytes, cancer-associated fibroblasts (CAFs), myofibroblasts and mesenchymal stem cells (MSCs), interact with CSCs directly or by secreting growth factors and cytokines.Citation1 Among stromal cells, tumor-associated macrophages (TAMs) majorly contribute to the tumor mass. High levels of tumor-infiltrating macrophages are often correlated with high tumor stage and poor disease outcome.Citation2 Although numerous studies have highlighted the links between TAMs and tumor initiation, proliferation, immunosuppression, angiogenesis and metastasis, the precise role of TAMs on the functions of CSCs remains largely unknown.
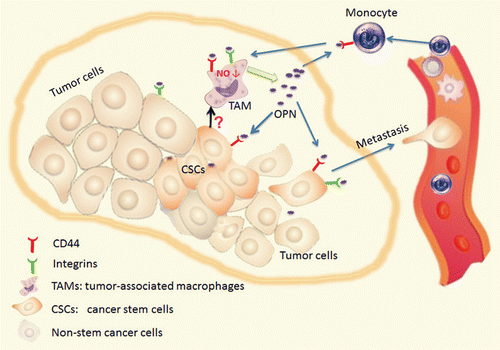
In a recent issue of Clinical Cancer Research, we have demonstrated that TAMs interact with CD44+ colorectal CSCs by secreting osteopontin (OPN), hence promoting their tumorigenicity and clonogenicity. Interestingly, we found that OPN secretion by TAMs is stimulated by CD44+ colorectal cancer cells and that the induction of OPN is closely associated with CD44 expression.Citation3 Although the exact mechanisms whereby CD44+ cancer cells stimulate OPN secretion are not clear, our data suggest that CSCs strongly rely on their niche and, at the same time, re-educate stromal cells (notably TAMs) to their own benefit.
OPN is a glycoprotein secreted by a variety of tissues, including bone, dental, renal and vascular tissues. OPN has been involved in a wide variety of cellular processes, such as atherosclerosis, bone remodeling, angiogenesis and wound healing.Citation4 OPN is also expressed by many types of tumors, including breast, lung, gastric, hepatic, colon and prostate carcinomas. Moreover, elevated levels of OPN in the tumor tissue or plasma have been correlated with poor disease outcome in cancer patients.Citation5 In many cases, the majority of cancer-associated OPN may be produced by stromal cells of the tumor microenvironment, especially TAMs and tumor-infiltrating leucocytes. Being a soluble protein, OPN produced by stromal cells can influence the activities of cancer cells both locally and at distant sites. Of note, OPN+ cancer cells are often found at the invasive tumor margin, in close proximity to stromal cells, indicating that OPN is involved in the paracrine interactions between malignant and stromal cells.Citation6 This might also explain the roles of OPN in tumor progression and metastasis. OPN secreted from both tumor cells and stromal cells can facilitate the adhesion, migration, homing, survival and proliferation of the former by binding to integrins and CD44 receptors.Citation4
The interactions between OPN and several distinct receptors can induce the activation of different signal transduction pathways, resulting in changes in the expression of multiple genes involved in cell survival, migration and invasion. For example, by binding to CD44, OPN has been shown enhance the formation of foci, invasion and tumorigenesis in H-Ras-V12-transformed cells via a RAC-mediated signal transduction cascade. OPN reportedly activates phosphoinositide-3-kinase (PI3K) to induce the phosphorylation of AKT through a αvβ3 integrin-transduced signaling pathway in breast cancer cells.Citation8 Furthermore, we observed that the OPN-CD44 interaction can increase the clonogenicity of colon cancer cells upon the elicitation of c-JUN N-terminal kinase (JNK)-transmitted signals, an effect that is abrogated by CD44-targeting blocking antibodies.Citation3
OPN not only exert tumor-promoting functions, but also operates as an immunoregulator within tumor microenvironment. Thus, OPN is able to recruit macrophages and neutrophils to the tumor site, and the RGD sequence of OPN is necessary for this function.Citation9 In many types of cancers, increased levels of OPN correlate with high levels of tumor-infiltrating leucocytes, including neutrophils and macrophages. Moreover, the binding of OPN to αvβ3 integrins has been described to affect nitric oxide production by macrophages, de facto decreasing their capability to kill tumor cells.Citation10 These observations highlight indirect effects whereby OPN promote tumor progression by recruiting immune cells to the tumor microenvironment.
Taken together, the results of the studies discussed above convincingly demonstrate a critical role for OPN in tumor progression (). The expression levels of OPN in tumor microenvironment are regulated by CSCs, and, in turn, OPN can affect the activity of CSCs by directly binding to distinct receptors expressed on the surface of cancer cells. In addition, the reciprocal interaction between OPN and CSCs can remodel the tumor microenvironment upon the recruitment of immune cells. These observations reveal that OPN functions as an important niche factor that links CSCs with their microenvironment. The development of therapies targeting OPN and OPN-related signaling networks holds promise for cancer therapy.
Figure 1. Osteopontin links CD44+ cancer stem cells with their microenvironment. CD44+ cancer stem cells stimulate the secretion of osteopontin (OPN) by tumor-associated macrophages (TAMs). Upon binding to integrins and CD44 expressed on the surface of several cell types, this results in decreased nitric oxide production by macrophages, in the activation of JNK-transduced signaling pathway in cancer cells as well as in the recruitment of monocytes and lymphocytes to the local microenvironment, overall favoring tumor progression.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Li L, Neaves WB. Normal stem cells and cancer stem cells: the niche matters. Cancer Res 2006; 66:4553 - 7; http://dx.doi.org/10.1158/0008-5472.CAN-05-3986; PMID: 16651403
- Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell 2010; 141:39 - 51; http://dx.doi.org/10.1016/j.cell.2010.03.014; PMID: 20371344
- Rao G, Wang H, Li B, Huang L, Xue D, Wang X, et al. Reciprocal interactions between tumor-associated macrophages and CD44-positive cancer cells via osteopontin/CD44 promote tumorigenicity in colorectal cancer. Clin Cancer Res 2013; 19:785 - 97; PMID: 23251004
- Rangaswami H, Bulbule A, Kundu GC. Osteopontin: role in cell signaling and cancer progression. Trends Cell Biol 2006; 16:79 - 87; http://dx.doi.org/10.1016/j.tcb.2005.12.005; PMID: 16406521
- Johnston NI, Gunasekharan VK, Ravindranath A, O’Connell C, Johnston PG, El-Tanani MK. Osteopontin as a target for cancer therapy. Front Biosci 2008; 13:4361 - 72; http://dx.doi.org/10.2741/3009; PMID: 18508515
- Anborgh PH, Mutrie JC, Tuck AB, Chambers AF. Role of the metastasis-promoting protein osteopontin in the tumour microenvironment. J Cell Mol Med 2010; 14:2037 - 44; http://dx.doi.org/10.1111/j.1582-4934.2010.01115.x; PMID: 20597997
- Teramoto H, Castellone MD, Malek RL, Letwin N, Frank B, Gutkind JS, et al. Autocrine activation of an osteopontin-CD44-Rac pathway enhances invasion and transformation by H-RasV12. Oncogene 2005; 24:489 - 501; http://dx.doi.org/10.1038/sj.onc.1208209; PMID: 15516973
- Das R, Mahabeleshwar GH, Kundu GC. Osteopontin stimulates cell motility and nuclear factor kappaB-mediated secretion of urokinase type plasminogen activator through phosphatidylinositol 3-kinase/Akt signaling pathways in breast cancer cells. J Biol Chem 2003; 278:28593 - 606; http://dx.doi.org/10.1074/jbc.M303445200; PMID: 12771144
- Atai NA, Bansal M, Lo C, Bosman J, Tigchelaar W, Bosch KS, et al. Osteopontin is up-regulated and associated with neutrophil and macrophage infiltration in glioblastoma. Immunology 2011; 132:39 - 48; http://dx.doi.org/10.1111/j.1365-2567.2010.03335.x; PMID: 20722758
- Wai PY, Guo L, Gao C, Mi Z, Guo H, Kuo PC. Osteopontin inhibits macrophage nitric oxide synthesis to enhance tumor proliferation. Surgery 2006; 140:132 - 40; http://dx.doi.org/10.1016/j.surg.2006.02.005; PMID: 16904962