Abstract
Retinoic acid-inducible gene I (RIG-I) is a pattern recognition receptor that is activated by 5′-triphosphate RNA molecules to induce type I interferon secretion and apoptosis in response to viral infection. We have designed a bifunctional small-interfering RNA that combines transforming growth factor β silencing with RIG-I activation to break tumor-induced immunosuppression. This strategy showed therapeutic efficacy in a murine model of pancreatic cancer.
Keywords: :
Tumors have evolved numerous mechanisms to promote their own growth and to evade immune attacks. A key cytokine mediating tumor growth, invasion, metastasis and angiogenesis is transforming growth factor β (TGFβ).Citation1 Importantly, TGFβ also plays a central role in tumor-induced immunosuppression, as it inhibits cytotoxic T lymphocytes (CTLs) and NK cells, stimulates regulatory T cells and shifts antigen-presenting cell functions toward the induction of tolerance.Citation2,Citation3 Tumor cells as well as immune cells including myeloid-derived suppressor cells (MDSC) and regulatory T cells contribute to TGFβ production and entertain a vicious circle of negative immune regulation. These attributes make TGFβ an interesting target for cancer immunotherapy.
Among multiple mechanisms, type I interferon (IFN) plays a central role in tumor immunosurveillance.Citation4 Therapeutically, IFNs function as multifaceted immune modulators, promoting TH1 responses and inhibiting immunosuppressive cells such as MDSCs and regulatory T cells. The induction of type I IFN with adjuvants including Toll-like receptor (TLR) ligands has therefore been recognized as a promising strategy for tumor immunotherapy. However, TLR expression is limited to some populations of immune cells. In contrast, retinoic acid-inducible gene I (RIG-I), a cytosolic sensor of viral RNA that detects 5′-triphosphate RNA species (ppp-RNA)Citation5,Citation6 is near-to-ubiquitously expressed. Recent evidence suggests that RIG-I represents a novel target for cancer immunotherapy.Citation7,Citation8 RIG-I initiates a signaling cascade involving interferon regulatory factor (IRF)3, IRF7 and NF-κB that leads to an antiviral response characterized by the production of type I IFN and other factors that sustain innate immunity.Citation9 Moreover, tumor cells appear to be susceptible to RIG-I-induced apoptosis, whereas their non-malignant counterparts are protected by a BCL-XL-dependent mechanism.Citation7 Thus, the administration of ppp-RNA to cancer patients might mimic a viral infection and initiate a type I IFN-mediated immune response that overcomes tumor-mediated immunosuppression.
Novel triphosphate small-interfering RNA (ppp-siRNA) strategies offer the advantage of combining RIG-I-mediated immune activation with RNA interference (RNAi)-mediated gene silencing within a single molecule (). This approach was first successfully applied in a murine melanoma model in which the anti-apoptotic molecule BCL-2 was chosen as a target for RNAi-mediated downregulation to promote tumor cell death.Citation8 However, little is known about the expression of RIG-I in cancers other than melanoma as well as on the ideal RNAi target to be used in strategies of this type. We hypothesized that dual targeting of tumor-mediated immunosuppression via RIG-I activation and TGFβ silencing might constitute an effective measure against pancreatic cancer, which is known to express high levels of TGFβ and hence to establish a particularly immunosuppressive tumor milieu.
Figure 1. Immune activation with a triphosphate small-interfering RNA targeting transforming growth factor β brakes tumor-induced immunosuppression. (A) A 5′-triphosphate-modified small-interfering RNA targeting transforming growth factor β (ppp-TGFβ) combines the potential of RNA-interference (RNAi)-mediated TGFβ silencing and that of retinoic acid-inducible gene I (RIG-I) activation, leading to the secretion of type I interferon (IFN) and other pro-inflammatory cytokines. (B) In vivo, the administration of ppp-TGFβ leads to the production of type I IFN and various chemokines (such as CXCL10), to the upregulation of MHC class I expression on tumor cells as well as to tumor cell apoptosis. Additionally, ppp-TGFβ favors the apoptotic demise of myeloid-derived suppressor cells (MDSCs), the recruitment of CD8+ T cells into neoplastic lesions, TH1 polarization and cytotoxic T lymphocyte (CTL) activation in a murine model of pancreatic carcinoma.
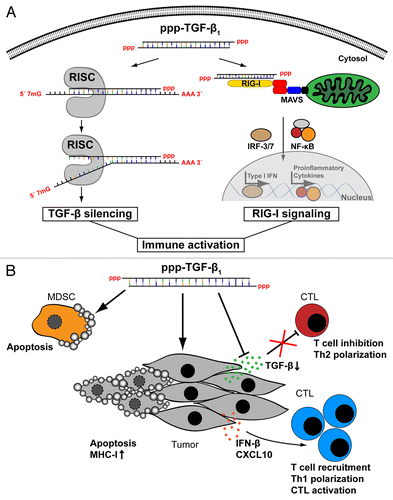
We have recently validated RIG-I as a therapeutic target for pancreatic cancer.Citation10 RIG-I expression was detected in all primary tumor samples and pancreatic cancer cell lines investigated in this respect. In line with this notion, activating RIG-I with ppp-RNA induced IRF3 phosphorylation, type I IFN secretion, as well as caspase-9-mediated apoptosis in pancreatic carcinoma cells. We next generated a ppp-modified siRNA targeting TGFβ (ppp-TGFβ) to combine RIG-I activation with the RNAi-mediated silencing of TGFβ and evaluated its therapeutic efficacy in a murine orthotopic model of pancreatic cancer. Our studies confirmed the dual activity of ppp-TGFβ, as (1) both the systemic and intratumoral levels of TGFβ were significantly reduced upon the administration of ppp-TGFβ, and (2) ppp-TGFβ induced RIG-I activation in vivo, resulting in high levels of type I IFN production, immune cell activation and consistent degrees of tumor cell death. Importantly, ppp-TGFβ significantly prolonged the survival of tumor-bearing mice and induced long-term tumor regression. In this regard, ppp-TGFβ was more effective than RNA molecules that contained either the RIG-I ligand motif or the TGFβ-silencing sequence alone.
We observed that the administration of ppp-TGFβ led to the recruitment of activated CD8+ T cells into tumors. Moreover, depletion of CD8+ T-cell abrogated the therapeutic efficacy of ppp-TGFβ, pointing to the emergence of a therapeutically relevant adaptive immune response against the tumor. In this regard, the finding that ppp-TGFβ reduced the amount of MDSCs, which are found in increased numbers in pancreatic cancer patients and potently suppress CD8+ T-cell functions, is of particular interest. Thus, the dual activity of ppp-TGFβ appear to have additive effects on breaking the immunosuppressive milieu established by pancreatic cancers, tipping the balance toward effective antitumor immune responses ().
An interesting aspect of ppp-TGFβ treatment is the induction of tumor cell death. In particular, we found that ppp-TGFβ triggers the mitochondrial pathway of apoptosis in pancreatic cancer cells, involving the upregulation of the BH3-only proteins NOXA and PUMA as well as caspase-9 activation. The systemic administration of ppp-TGFβ did not affect the normal pancreas or other organs, such as the lung, liver and kidneys, confirming previous reports that tumor cells are highly susceptible to ppp-RNA-induced apoptosis.Citation7,Citation8 Such a preferential killing of malignant cells may thus provide a therapeutic window for the clinical implementation of ppp-RNA-based anticancer immunotherapies.
A major advantage of the ppp-siRNA technology is its versatility, as the silencing target can be adapted to specific tumor entities or even to the characteristics of individual neoplasms. Moreover, at least hypothetically, several siRNA targets can be combined to synergistically inhibit tumor cell survival, proliferation, metastasis, angiogenesis, reprogrammed metabolism and immunosuppression. In addition, adjuvants enhancing the sensitivity of tumor cells to RIG-I signaling, such as IFNα, could be employed to increase the therapeutic efficacy of ppp-siRNAs. A strategy that is currently explored by our group is the combination of ppp-siRNA with chemotherapy. Further advances in the field can be expected by the development of siRNA delivery systems that specifically target malignant cells. In conclusion, the therapeutic potential of bifunctional ppp-siRNA is just beginning to be unraveled. The versatility of this technology offers a wide range of applications for different tumor entities.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Massagué J. TGFbeta in Cancer. Cell 2008; 134:215 - 30; http://dx.doi.org/10.1016/j.cell.2008.07.001; PMID: 18662538
- Moutsopoulos NM, Wen J, Wahl SM. TGF-beta and tumors--an ill-fated alliance. Curr Opin Immunol 2008; 20:234 - 40; http://dx.doi.org/10.1016/j.coi.2008.04.003; PMID: 18486463
- Yang L, Pang Y, Moses HL. TGF-beta and immune cells: an important regulatory axis in the tumor microenvironment and progression. Trends Immunol 2010; 31:220 - 7; http://dx.doi.org/10.1016/j.it.2010.04.002; PMID: 20538542
- Dunn GP, Bruce AT, Sheehan KC, Shankaran V, Uppaluri R, Bui JD, et al. A critical function for type I interferons in cancer immunoediting. Nat Immunol 2005; 6:722 - 9; http://dx.doi.org/10.1038/ni1213; PMID: 15951814
- Hornung V, Ellegast J, Kim S, Brzózka K, Jung A, Kato H, et al. 5′-Triphosphate RNA is the ligand for RIG-I. Science 2006; 314:994 - 7; http://dx.doi.org/10.1126/science.1132505; PMID: 17038590
- Schmidt A, Schwerd T, Hamm W, Hellmuth JC, Cui S, Wenzel M, et al. 5′-triphosphate RNA requires base-paired structures to activate antiviral signaling via RIG-I. Proc Natl Acad Sci U S A 2009; 106:12067 - 72; http://dx.doi.org/10.1073/pnas.0900971106; PMID: 19574455
- Besch R, Poeck H, Hohenauer T, Senft D, Häcker G, Berking C, et al. Proapoptotic signaling induced by RIG-I and MDA-5 results in type I interferon-independent apoptosis in human melanoma cells. J Clin Invest 2009; 119:2399 - 411; PMID: 19620789
- Poeck H, Besch R, Maihoefer C, Renn M, Tormo D, Morskaya SS, et al. 5′-Triphosphate-siRNA: turning gene silencing and Rig-I activation against melanoma. Nat Med 2008; 14:1256 - 63; http://dx.doi.org/10.1038/nm.1887; PMID: 18978796
- Yoneyama M, Fujita T. RNA recognition and signal transduction by RIG-I-like receptors. Immunol Rev 2009; 227:54 - 65; http://dx.doi.org/10.1111/j.1600-065X.2008.00727.x; PMID: 19120475
- Ellermeier J, Wei J, Duewell P, Hoves S, Stieg MR, Adunka T, et al. Therapeutic efficacy of bifunctional siRNA combining TGF-beta1 silencing with RIG-I activation in pancreatic cancer. Cancer Res 2013; In press http://dx.doi.org/10.1158/0008-5472.CAN-11-3850; PMID: 23338611