Abstract
The communication between tumor and host cells involves signals that act across extended distances in the body. Recent evidence indicates that the hormone angiotensin II is overproduced by lung adenocarcinoma to remotely expand bone marrow-derived hematopoietic stem cells. This process amplifies the supply of tumor-associated macrophages, which promote disease progression.
Malignant cells are known to co-opt seemingly normal immune cells, such as tumor-associated macrophages (TAMs), to foster tumor growth and progression.Citation1 Large numbers of TAMs frequently accumulate in the tumor stroma, and these cells can promote malignant progression by stimulating invasiveness, angiogenesis and metastasis as well as by suppressing antitumor immune responses. In addition, retrospective studies of patients affected by different types of cancer, including breast, bladder, ovarian, prostate and non-small cell lung carcinomas, indicate that elevated TAM densities are associated with poor overall survival. Thus, TAMs constitute candidate therapeutic targets for the treatment of various types of cancer.
Macrophages are terminally differentiated cells that may not proliferate in the tumor stroma. Thus, as they turn over within a few days, TAMs must be constantly replaced throughout cancer progression. In line with this notion, recent studies indicate that TAMs are continuously replenished in vivo, mostly by circulating inflammatory Ly-6Chi monocytes, which are cells that foster tumor progression.Citation2,Citation3 These findings suggest that tumors can act locally to promote the recruitment of immune cells that are normally present in the circulation.
Additional evidence indicates that some cancers alter the hematopoietic system more profoundly, by remotely instigating the amplification of cells that promote tumor progression once recruited to the tumor microenvironment. Indeed, the ontogenic analysis of TAMs in a murine model of lung adenocarcinoma (driven by the activation of oncogenic Kras coupled to the inactivation of p53, called KP) has revealed that cancer amplifies hematopoietic stem cells (HSCs) and instigates the expansion of macrophagic progenitors.Citation3 This process predominantly occurs outside the bone marrow and within the splenic monocyte reservoir.Citation4
Tumor-induced extramedullary progenitor activity seems to develop equally well in mice and humans, at least in individuals affected by invasive tumors.Citation3 Indeed, the splenocytes of patients manifesting pathological evidence of invasive cancer can contain high amounts of lineage-negative CD117+CD34+CD38+IL3Ra+CD45RA+ cells, which resemble bone marrow myeloid progenitor cells. As expected, these cells are also PU.1+CEBPe+G-CSFR+GM-CSFR+MPO+GATA1−GATA3−vWF−IL7Ra− and differentiate into macrophages in vitro as well as when administered to immunodeficient mice bearing human lung adenocarcinoma xenografts.Citation3 When taking scale differences between species into account, the magnitude of splenic macrophage progenitor amplification in mice and humans appears to be comparable.Citation3 This amplification occurs in the absence of a notable expansion of splenic tissue, at least in KP mice. In contrast, mice engrafted with tumor cell lines often show enlarged spleens.
Adoptive transfer and splenectomy experiments have confirmed that a large fraction of TAMs can descend from splenic macrophage progenitors. Moreover, the accumulation of macrophage precursors in the spleen can promote tolerance toward tumor-associated antigens.Citation5 These findings suggest that (at least some) tumors have the ability to remotely interfere with the mononuclear phagocyte lineage in ways that favor tumor progression.
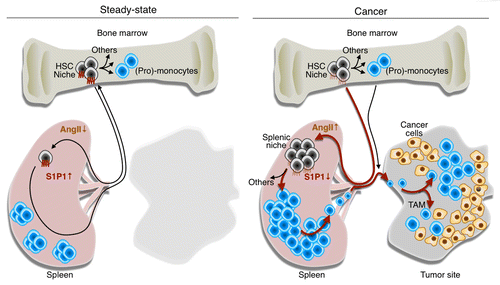
Identifying the factors whereby malignant cells remotely control tumor-promoting macrophagic responses may provide novel targets for anticancer therapy. Interestingly, tumor-bearing KP mice exhibit increased circulating levels of the peptide hormone angiotensin II (AngII).Citation6 In addition, the continuous delivery of AngII to otherwise healthy mice is sufficient to phenocopy the tumor-induced splenic amplification of macrophage progenitors.Citation6 The response mediated by AngII is independent of hemodynamic changes (blood pressure does not increase in tumor-bearing KP mice), selectively requires the expression of the AngII receptor AGTR1A on HSCs and downregulates the sphingosine-1-phosphate receptor 1 (S1P1) signaling pathway in HSCs.Citation6 This latter signal transduction cascade is essential for controlling the egression of HSCs from peripheral lymphoid tissues. Taken together, these data identify AngII as a prototypical signaling molecule that can be overproduced by cancer and act in an endocrine manner to actively stimulate the production of tumor-supporting cells ().
Figure 1. Tumor remote control of hematopoietic stem cells and macrophagic progenitors through angiotensin II. In steady-state conditions, hematopoietic stem cells (HSCs) and macrophagic progenitors typically reside in bone marrow niches and generate (pro-)monocytes. A small number of HSCs constantly enter the circulation, extravasate at distant sites and migrate through peripheral tissues, including the spleen. Under normal conditions, these cells can re-enter the blood and ultimately, return to their niches in the bone marrow. The process of cell recirculation is controlled at least in part by a chemotactic gradient of sphingosine-1-phosphate (S1P) that is established between peripheral tissues, lymphatics and the blood, where S1P concentrations is the highest. Thus, in steady-state conditions, only a few HSCs can be found in the spleen. Multiple types of cancer including lung adenocarcinomas produce angiotensin II (AngII), which directly signals through the AGTR1A receptor expressed on HSCs. This induces the downregulation of the S1P receptor 1 (S1P1), hence reducing the ability of HSCs to sense the S1P gradient. In these conditions, HSCs accumulate in the spleen where they give rise to monocytes. By continuously producing monocytes, the spleen can contribute TAMs throughout tumor progression. Thus, tumors can directly exploit the endocrine system to promote tumor progression. Drugs that interfere with the AngII pathway (or perhaps with S1P1 signaling) may hence become therapeutic options for treating lung cancer patients in whom this pathway is elevated.
AngII signaling can be efficiently suppressed by administering either angiotensin-converting enzyme inhibitors (ACEis) or AngII receptor blockers (ARBs). Both the ACEi enalapril and the ARB losartan restore S1P1 expression in the HSCs of tumor-bearing KP mice. Consequently, enalapril treatment prevents the amplification of both HSCs and macrophagic progenitors, restrains the TAM response and increases mouse survival (losartan has not been tested in this respect).Citation6 The survival gain obtained with ACEi is equivalent to that observed with standard-of-care chemotherapy.
Some retrospective clinical studies have begun to explore whether ACEis or ARBs may control cancer incidence and cancer-related death.Citation7-Citation9 Although these studies investigated cancer patients regardless of their AngII profile and TAM content, some of them suggest that ACEis or ARBs might reduce the incidence of specific malignancies such as lung and breast cancer. The putative antitumor effects of ACEis may depend on mechanisms other than those controlling blood pressure, as the risk of developing a tumor apparently does not decrease in patients receiving other classes of antihypertensive drugs.Citation7
At present, well-controlled prospective studies are needed to address whether ACEis or ARBs may be used to improve disease outcomes in selected cancer patient cohorts (e.g., patients exhibiting increased circulating levels of AngII). Increasing evidence suggests that TAMs modulate the efficacy of anticancer therapy. Thus, controlling TAM production with agents such as ACEis may also be useful to design more effective combination therapies. Initial gene expression profiling studies suggest that angiotensinogen (the precursor of AngII) is frequently overexpressed by tumor tissues, at least in lung and breast cancer patients (ref. Citation6 and unpublished data). However, only a subset of these patients might upregulate the AngII signaling pathway.Citation6 As hypertension may not necessarily indicate increased circulating levels of AngII,Citation10 patient selection may require a direct quantification of AngII concentrations in the blood.
The control operated by tumors on the host immune system extends well beyond the local microenvironment. Identifying tumor-derived long-range factors that are capable of amplifying tumor-supporting immune cells may pave the way for new targeted anticancer regimens. AngII is one of such factor. However, tumors most likely exert a remote control on hematopoietic cells through a wide array of mediators, the majority of which remain to be discovered.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell 2010; 141:39 - 51; http://dx.doi.org/10.1016/j.cell.2010.03.014; PMID: 20371344
- Movahedi K, Laoui D, Gysemans C, Baeten M, Stangé G, Van den Bossche J, et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res 2010; 70:5728 - 39; http://dx.doi.org/10.1158/0008-5472.CAN-09-4672; PMID: 20570887
- Cortez-Retamozo V, Etzrodt M, Newton A, Rauch PJ, Chudnovskiy A, Berger C, et al. Origins of tumor-associated macrophages and neutrophils. Proc Natl Acad Sci U S A 2012; 109:2491 - 6; http://dx.doi.org/10.1073/pnas.1113744109; PMID: 22308361
- Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 2009; 325:612 - 6; http://dx.doi.org/10.1126/science.1175202; PMID: 19644120
- Ugel S, Peranzoni E, Desantis G, Chioda M, Walter S, Weinschenk T, et al. Immune tolerance to tumor antigens occurs in a specialized environment of the spleen. Cell Rep 2012; 2:628 - 39; http://dx.doi.org/10.1016/j.celrep.2012.08.006; PMID: 22959433
- Cortez-Retamozo V, Etzrodt M, Newton A, Ryan R, Pucci F, Sio SW, et al. Angiotensin II Drives the Production of Tumor-Promoting Macrophages. Immunity 2013; 38:296 - 308; http://dx.doi.org/10.1016/j.immuni.2012.10.015; PMID: 23333075
- Lever AF, Hole DJ, Gillis CR, McCallum IR, McInnes GT, MacKinnon PL, et al. Do inhibitors of angiotensin-I-converting enzyme protect against risk of cancer?. Lancet 1998; 352:179 - 84; http://dx.doi.org/10.1016/S0140-6736(98)03228-0; PMID: 9683206
- Bangalore S, Kumar S, Kjeldsen SE, Makani H, Grossman E, Wetterslev J, et al. Antihypertensive drugs and risk of cancer: network meta-analyses and trial sequential analyses of 324,168 participants from randomised trials. Lancet Oncol 2011; 12:65 - 82; http://dx.doi.org/10.1016/S1470-2045(10)70260-6; PMID: 21123111
- Huang CC, Chan WL, Chen YC, Chen TJ, Lin SJ, Chen JW, et al. Angiotensin II receptor blockers and risk of cancer in patients with systemic hypertension. Am J Cardiol 2011; 107:1028 - 33; http://dx.doi.org/10.1016/j.amjcard.2010.11.026; PMID: 21256465
- Walker WG, Whelton PK, Saito H, Russell RP, Hermann J. Relation between blood pressure and renin, renin substrate, angiotensin II, aldosterone and urinary sodium and potassium in 574 ambulatory subjects. Hypertension 1979; 1:287 - 91; http://dx.doi.org/10.1161/01.HYP.1.3.287; PMID: 399240