Abstract
We identified a critical role for receptor for advanced glycation end products (RAGE) in the intratumoral accumulation of myeloid-derived suppressor cells (MDSCs) during pancreatic carcinogenesis. The absence of RAGE markedly delayed neoplasia and limited MDSC accumulation in mice expressing an oncogenic variant of Kras. In spite of MDSCs, these mice accumulated non-immunosuppressive macrophages. Thus, RAGE regulates carcinogenesis and consequent myeloid responses.
Keywords: :
Pancreatic cancer is notoriously difficult to treat and exhibit many hallmarks of immunosuppression. The prevention of successful immune responses against pancreatic neoplasms is accomplished (at least in part) by the recruitment and retention of myeloid-derived suppressor cells (MDSCs).Citation1 This heterogeneous cell population directly inhibits the activities of immune effector cells through several mechanisms, including nutrient deprivation and the production of reactive nitrogen and oxygen species. While MDSC are difficult to phenotypically characterize in humans, they can readily and reliably be identified in mice through the co-expression of CD11b and Gr1.Citation2
Damage associated molecular patterns (DAMPs) serve as inflammatory signals that alert the host to cellular and tissue injury.Citation3 DAMPs and their cognate receptors shape the nature of both the innate and adaptive immune response, in some cases exerting potent adjuvant effects while promoting tissue repair in others.Citation3 The receptor for advanced glycation end products (RAGE, also known as AGER) is a pattern-recognition receptor that recognizes several DAMPs including high-mobility group box 1 (HMGB1) and S100A8/A9.Citation4 In physiological conditions, RAGE is expressed in the lung and in cells of the immune system. In addition, RAGE is overexpressed by many carcinomas, including pancreatic ductal adenocarcinoma.Citation4-Citation6 Studies from our group and others have demonstrated a crucial role for RAGE in sustaining tumor growth via the establishment of a pro-inflammatory microenvironment and via the release of interleukin-6 (IL-6).Citation4
Previous studies have implicated S100A8/A9 in the recruitment and retention of MDSCs in murine models of colorectal cancer, and the peripheral levels of these proteins correlate with the frequency of MDSCs in the serum of patients bearing gastric cancers.Citation7 We sought to determine whether RAGE regulates the myeloid response to early pancreatic carcinogenesis using a murine model of oncogenic Kras-driven pancreatic cancer (KC mice).Citation8 These mice were backcrossed into an Ager null background to generate animals that would develop pancreatic tumors in the absence of the receptor (KCR mice).
Strikingly, in the absence of RAGE pancreatic carcinogenesis proceeded at a slower rate and MDSCs failed to accumulate, both systemically and within the tumor microenvironment (TME) (). While RAGE itself was not required for MDSC development, the phenotype and function of the myeloid cells (CD11b+) isolated from KCR mice and KC mice were distinct. At comparable ages, CD11b+ cells isolated from the spleen and pancreas of KCR mice expressed F4/80 (a marker of mature macrophages), rather than Gr1, and were indeed non-immunosuppressive in vitro.Citation9 It is currently unclear whether the apparent lack of MDSCs in the KCR mouse strain directly contributes to delayed carcinogenesis or whether, vice versa, their absence is a consequence of inhibited tumor growth stemming from the ablation of Ager.
Figure 1. RAGE signaling recruits myeloid-derived suppressor cells and dendritic cells. Receptor for advanced glycation end products (RAGE) ligands such as high mobility group box 1 HMGB1 and S100A8/A9 are actively secreted or passively released in the tumor microenvironment by stressed and dying malignant cells as well as by specific immune cells. The activation of RAGE, which is often overexpressed on transformed cells, cause the expression of chemokines involved in angiogenesis and tissue repair while favoring the intratumoral accumulation of myeloid-derived suppressor cells (MDSCs). In the absence of RAGE, MDSCs fail to accumulate and are replaced by mature macrophage-like cells, overall resulting in the inhibition of carcinogenesis. DC, dendritic cell.
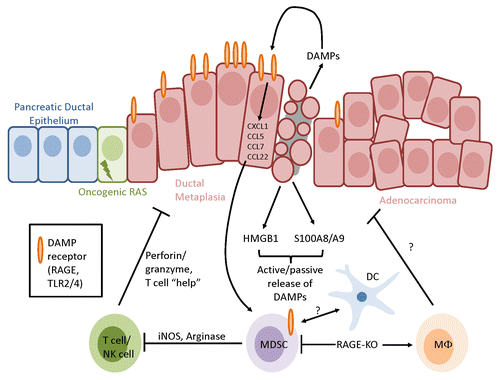
Several explanations may be put forward to explain the failure of KCR mice to accumulate MDSCs, including a diminished longevity or enhanced rates of apoptosis affecting this cell compartment. However, given the lower levels of CCL22 in the pancreatic TME of KCR mice and since an increased production of CXCL1, CCL5 and CCL7 by malignant cells has previously been shown to stem from RAGE activation, it is likely that the recruitment of MDSCs is specifically disrupted in the absence of RAGE.Citation2,Citation5 Also the precise role of the CD11b+F4/80+Gr1− cells that accumulate in the spleens and pancreata of KCR mice is unknown (). Although these cells are not immunosuppressive, future studies will determine whether they have direct antitumor effects. Macrophages have indeed been demonstrated to be critical for antitumor immunity mediated by CD40 agonism.Citation10 Finally, it remains to be understood in which cell compartment RAGE signaling is required for the accumulation of MDSCs. Three distinct possibilities include the tumor itself, the stroma and the bone marrow. While it is experimentally difficult to distinguish between malignant cells and their stroma, the involvement of the immune compartment in this phenomenon can readily be assessed by the transplantation of wild-type or Ager−/− bone marrow into KC recipients. These studies are ongoing.
As we appreciate more deeply the significant role that immunosuppressive immune cells such as MDSCs play in carcinogenesis, it becomes very important to understand which receptors on the surface of cells and malignant cells mediate their recruitment and retention into the TME. Importantly, the identification of immunoregulatory roles for proteins such as RAGE converts them in candidate targets for inhibiting tumor-promoting myeloid responses in cancer patients.
| Abbreviations: | ||
| DAMP | = | damage-associated molecular pattern |
| HMGB1 | = | high-mobility group box 1 |
| IL-6 | = | interleukin-6 |
| MDSC | = | myeloid-derived suppressor cell |
| RAGE | = | receptor for advanced glycation end-products |
| TME | = | tumor microenvironment |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Clark CE, Hingorani SR, Mick R, Combs C, Tuveson DA, Vonderheide RH. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res 2007; 67:9518 - 27; http://dx.doi.org/10.1158/0008-5472.CAN-07-0175; PMID: 17909062
- Ichikawa M, Williams R, Wang L, Vogl T, Srikrishna G. S100A8/A9 activate key genes and pathways in colon tumor progression. Mol Cancer Res 2011; 9:133 - 48; http://dx.doi.org/10.1158/1541-7786.MCR-10-0394; PMID: 21228116
- Lotze MT, Zeh HJ, Rubartelli A, Sparvero LJ, Amoscato AA, Washburn NR, et al. The grateful dead: damage-associated molecular pattern molecules and reduction/oxidation regulate immunity. Immunol Rev 2007; 220:60 - 81; http://dx.doi.org/10.1111/j.1600-065X.2007.00579.x; PMID: 17979840
- Kang R, Loux T, Tang D, Schapiro NE, Vernon P, Livesey KM, et al. The expression of the receptor for advanced glycation endproducts (RAGE) is permissive for early pancreatic neoplasia. Proceedings of the National Academy of Sciences 2012; http://dx.doi.org/10.1073/pnas.1113865109
- Muthuswamy R, Berk E, Junecko BF, Zeh HJ, Zureikat AH, Normolle D, et al. NF-κB hyperactivation in tumor tissues allows tumor-selective reprogramming of the chemokine microenvironment to enhance the recruitment of cytolytic T effector cells. Cancer Res 2012; 72:3735 - 43; http://dx.doi.org/10.1158/0008-5472.CAN-11-4136; PMID: 22593190
- Kang R, Tang D, Schapiro NE, Livesey KM, Farkas A, Loughran P, et al. The receptor for advanced glycation end products (RAGE) sustains autophagy and limits apoptosis, promoting pancreatic tumor cell survival. Cell Death Differ 2010; 17:666 - 76; http://dx.doi.org/10.1038/cdd.2009.149; PMID: 19834494
- Wang L, Chang EWY, Wong SC, Ong S-M, Chong DQY, Ling KL. Increased myeloid-derived suppressor cells in gastric cancer correlate with cancer stage and plasma S100A8/A9 proinflammatory proteins. J Immunol 2013; 190:794 - 804; http://dx.doi.org/10.4049/jimmunol.1202088; PMID: 23248262
- Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 2003; 4:437 - 50; http://dx.doi.org/10.1016/S1535-6108(03)00309-X; PMID: 14706336
- Vernon PJ, Loux TJ, Schapiro NE, Kang R, Muthuswamy R, Kalinski P, et al. The receptor for advanced glycation end products promotes pancreatic carcinogenesis and accumulation of myeloid-derived suppressor cells. J Immunol 2013; 190:1372 - 9; http://dx.doi.org/10.4049/jimmunol.1201151; PMID: 23269246
- Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 2011; 331:1612 - 6; http://dx.doi.org/10.1126/science.1198443; PMID: 21436454