Abstract
Ovarian cancer is one of the neoplasms affecting the reproductive tract associated with high mortality rate because of limited therapeutic options and an elevated incidence of chemoresistance and recurrence. In this context, immunotherapy may constitute a promising approach to improve survival rates and clinical outcome, raising the need for specific target antigens. Cancer-testis antigens (CTAs) are considered promising candidates in this sense because they are aberrant expressed by various malignancies but not by non-transformed tissue, with the exception of testes. Here, we examined the expression and potential to promote humoral immune responses of a novel CTA, A-kinase anchor protein 4 (AKAP4), among 38 ovarian carcinoma patients. Our results reveal that AKAP4 was expressed at both the mRNA and protein levels in 89% (34/38) of ovarian carcinoma tissue specimens but not in 21 matched adjacent non-cancerous tissues. In addition, a humoral response against AKAP4 was detected in 58% (22/38) of ovarian carcinoma patients by ELISA. In particular, 65% (22/34) patients bearing an AKAP4-expressing tumor exhibited circulating anti-AKAP4 antibodies. Interestingly, the majority of specimens were categorized as ovarian serous adenocarcinoma and serous papillary carcinoma, of which 93% (28/30) and 100% (6/6), respectively, expressed AKAP4. A humoral response against AKAP4 was detected in 79% (19/24) and 67% (4/6) of ovarian serous adenocarcinoma and serous papillary carcinoma patients, respectively. The presence of circulating anti-AKAP4 antibodies suggests the AKAP4 is highly immunogenic in ovarian serous carcinoma patients. Our study lays the foundations for exploring AKAP4 as a potential target for the immunotherapy of ovarian cancer.
Introduction
Ovarian cancer is one of the gynecological malignancies associated with worst prognosis. In 2010, 21,888 women were estimated to receive a diagnosis of ovarian cancer in the United States and 13,850 deaths to occur because of ovarian cancer.Citation1 Ovarian serous carcinoma is the most prevalent (epithelial) subtype of ovarian cancer and accounts for about 80–90% of all ovarian cancers. As many other malignancies, early-stage ovarian cancers are often asymptomatic and hence are diagnoses often are made at an advanced disease stage, when therapeutic modalities are limited. Therefore, there is a need to explore alternative approaches to improve the clinical management of ovarian cancer patients, including immunotherapeutic strategies such as tumor-associated antigen-targeting vaccines, dendritic cell-based therapies, monoclonal antibody-based approaches and passive immunotherapy.Citation2
Immunotherapy has emerged as a promising treatment modality over standard chemotherapy because it is generally associated with reduced side effects and hence an improved quality of life for patients. In this regard, cancer-testis antigens (CTAs) have been indicated as a potential targets for antigen-specific immunotherapy because they are not found in somatic tissues except testes but are highly expressed by various malignancies and often trigger humoral immune responses in cancer patients.Citation2 One of the best-characterized CTA, sperm-associated antigen 9 (SPAG9) is expressed by multiple neoplasms but not in adjacent non-cancerous tissues (ANCTs).Citation2 Interestingly, humoral responses against SPAG9 were demonstrated in 67% (20/30) of epithelial ovarian cancer patients,Citation3 77% (40/52) of renal cell carcinoma patients,Citation4 80% (80/100) of breast cancer patients,Citation5 80% (54/66) of cervical cancer patients,Citation6 78% (92/118) of thyroid cancer patients,Citation7 88% (106/120) of chronic myeloid leukemia patients,Citation8 70% (38/54) of colorectal cancer patientCitation9 and 72% (36/50) of endometrial cancer patients investigated in this respect.Citation10 The immunogenicity of CTAs can be explained by the lack of MHC class I expression on the surface of germ cells, which de facto renders testes an immunoprivileged site.Citation11 Moreover, the education of T cells takes place in the thymus during early embryonic development, whereas spermatogenesis only starts once the puberty is attained. By that time, the immune system has refined its ability to distinguish “self” from “non-self.” As a result, new antigens expressed during spermatogenesis in the testes have never been encountered by the immune system and could be immunogenic. This renders CTAs, which are aberrantly expressed by various cancers, optimal candidate targets for cancer immunotherapy.
In ovarian cancer patients, the expression as well the presence of humoral or cellular immune responses have been documented only for a few CTAs, namely NY-ESO-1,Citation12 LAGECitation12 and sperm protein 17 (SP17).Citation13-Citation15 However, NY-ESO-1, LAGE and SP17 are generally expressed to low levels by ovarian tumors and are poorly immunogenicity, raising the need to identify and characterize novel CTAs. An ideal candidate target for immunotherapy should indeed be immunogenic, expressed to high levels by tumor cells (and not, or to very low levels by normal tissues) and localize to cell surface. Here, we explored the possible clinical utility of a new CTA, A-kinase anchor protein 4 (AKAP4), which we identified based on these criteria.Citation16,Citation17 We examined the expression of AKAP4 at the mRNA and protein levels in various ovarian carcinoma specimens by RT-PCR, in situ hybridization and immunohistochemistry, revealing that AKAP4 was expressed in 89% (34/38) of ovarian tissue specimens but not in matched ANCTs. Further, we detected an anti-AKAP4 humoral response in 58% of ovarian carcinoma patients, irrespective of disease stage and subtype. This study lays the foundations for exploring AKAP4 as a novel target for the immunotherapy of ovarian cancer.
Results
AKAP4 is expressed in ovarian serous carcinoma tissue specimens
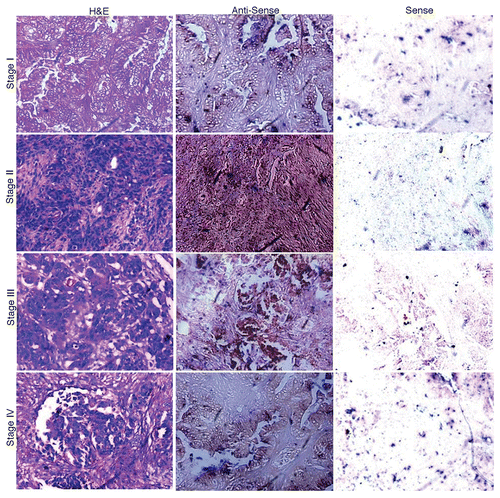
Our RT-PCR analysis revealed AKAP4 mRNA expression in 89% (34/38) of all ovarian carcinoma samples investigated in this sense but not in matched ANCTs. The detection of AKAP4 transcripts in all stages (Stage I, Stage II, Stage III and Stage IV) of ovarian carcinoma and in testes is shown in . AKAP4 expression was detected in 93% (28/30) serous adenocarcinomas and 100% (6/6) serous papillary carcinomas. The sole endometroid adenocarcinoma specimen (representing a relatively rare clinical sub-type of ovarian cancer) that was available exhibited no AKAP4 expression. Along similar lines, no AKAP4 expression was observed in a benign tumor (adenofibroma). Of note, AKAP4 transcripts were detected in 100% (7/7) Stage I, 71% (5/7) Stage II, 85% (11/13) Stage III and 100% (11/11) Stage IV tissue specimens (). Localization studies of AKAP4 expression by in situ RNA hybridization revealed a chocolate brown reactivity upon hybridization with anti-sense riboprobes (). As expected, sense riboprobes failed to hybridize to tissue specimens.
Figure 1.AKAP4 expression in representative Stage I, Stage II, Stage III and Stage IV ovarian cancer tissue specimens. Tissue samples were analyzed by RT-PCR with AKAP4-specific primers. No AKAP4 expression was detected in matched adjacent non-cancerous tissue (ANCT) specimens. Testis cDNA was used as a positive control, while β actin was quantified as an internal loading control.
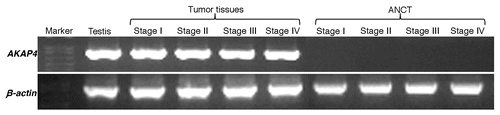
Table 1. AKAP4 expression and humoral response in ovarian carcinoma patients
Figure 2. In situ localization of AKAP4 mRNA in representative Stage I, Stage II, Stage III and Stage IV ovarian cancer tissue specimens. Ovarian cancer samples representative of all FIGO stages were stained with hematoxylin and eosin (left panels) or with AKAP4-specific anti-sense (middle panels) or sense (right panels) riboprobes, the latter as a negative control condition. Original magnification = 200×, = objective 20×.
Validation of AKAP4 protein expression in ovarian serous carcinoma tissue specimens
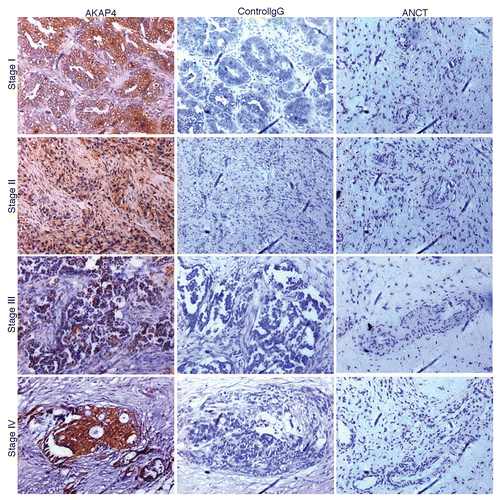
Immunohistochemical analyses were performed to validate the expression of AKAP4 at the protein level. Interestingly, there was no discrepancy between AKAP4 expression at the mRNA and protein levels. AKAP4 was indeed expressed in 89% (34/38) of ovarian carcinoma specimens but not in matched ANCTs. In particular, the AKAP4 protein was detected in 93% (28/30) serous adenocarcinoma and 100% (6/6) serous papillary carcinoma samples. Moreover, we failed to detect AKAP4 by immunohistochemistry in one endometroid adenocarcinoma and one adenofibroma specimens. Similar to what observed at the mRNA level, 100% (7/7) Stage I, 71% (5/7) Stage II, 85% (11/13) Stage III and 100% (11/11) Stage IV tissue specimens were positive for AKAP4 protein expression (; ). Neither ovarian cancer samples probed with unspecific, control IgGs nor ANCT specimens probed with polyclonal anti-AKAP4 antibodies stained positively for AKAP4 expression.
Figure 3. AKAP4 expression in representative Stage I, Stage II, Stage III and Stage IV tissue ovarian cancer specimens. Serial sections from ovarian cancer samples representative of all FIGO stages (left and middle panels) or from matched adjacent non-cancerous tissue (ANCT) specimens (right panel) were probed with polyclonal anti-AKAP4 antibodies (left and right panels) or with non-specific IgGs (central panel), as a staining control condition.
Anti-AKAP4 humoral responses in ovarian serous carcinoma patients
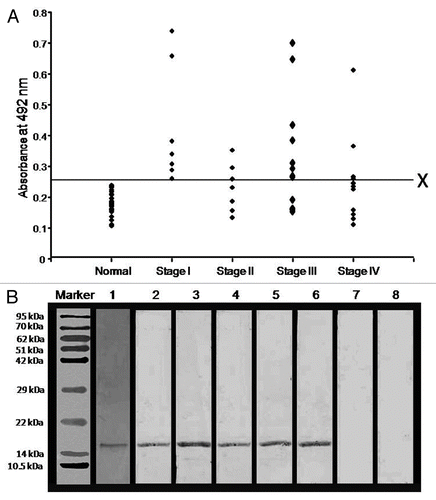
Humoral responses against AKAP4 were examined in the serum of 38 ovarian carcinoma patients and 25 healthy donors by a conventional ELISA-based approach. The mean + 2SD (0.257) of antibody titers of healthy donors was used as a cut-off value above which samples would considered positive for anti-AKAP4 humoral responses. Circulating anti-AKAP4 antibodies were detected in 58% (22/38) of all ovarian carcinoma patients. In particular, the sera from 79% (19/24) serous adenocarcinoma and 67% (4/6) serous papillary carcinoma patients were found to contain anti-AKAP4 antibodies. No anti-AKAP4 humoral responses were observed in the serum of one endometroid adenocarcinoma and one benign adenofibroma patient. Anti-AKAP4 antibodies were found in patients representative of all ovarian serous carcinoma FIGO stages, including 100% (7/7) Stage I, 43% (3/7) Stage II, 61% (8/13) Stage III and 36% (4/11) Stage IV tumors (). The presence of circulating anti-AKAP4 antibodies was confirmed by immunoblotting, de facto validating the results of ELISA tests (, Lane 3–6). Thus, the sera of healthy donors exhibited no immunoreactivity (, Lane 7). Finally, the specificity of patient-derived anti-AKAP4 antibodies was confirmed by means of a neutralization experiment. To this aim, the patient serum was pre-incubated with 15 μg/mL recombinant AKAP4 protein, resulting in the complete loss of immunoreactivity (, Lane 8).
Figure 4. Humoral responses against AKAP4 in ovarian carcinoma patients. (A) Figure Humoral responses against AKAP4 in ovarian cancer specimens obtained from Stage I, Stage II, Stage III and Stage IV patients. X represents the cut-off value (calculated as the mean of antibody titers detected in healthy donors + 2SD) above which all specimens were considered as positive. (B) Immunoblotting experiments were performed to confirm anti-AKAP4 humoral responses and the specificity of circulating anti-AKAP4 antibodies was validated by neutralization experiments. Lane 1, coomassie brilliant blue stained purified recombinant AKAP4 protein; Lane 2, polyclonal anti-AKAP4 antibody showing AKAP4 immunoreactivity; Lane 3–6, AKAP4-immunoreactive bands indicative of AKAP-specific humoral responses in representative stage I, stage II, stage III and stage IV patients; Lane 7, absence of anti-AKAP4 humoral responses in the serum of a healthy donor; Lane 8, absence of AKAP4-immunoreactive bands upon the pre-incubation of the patient's serum with 15 μg/mL recombinant AKAP4.
Discussion
Ovarian cancer remains among the leading causes of cancer-related mortality worldwide.Citation1 It is asymptomatic at early stages and hence diagnosed most often at advanced stages, when the therapeutic options are limited. Thus, novel therapeutic strategies are needed that overcome the poor response to chemotherapy and frequent recurrence of ovarian cancer.Citation18 In this context, immunotherapeutic regimens including antigen-specific, dendritic cell-based, adoptive cell transfer-based immunotherapy and monoclonal antibody-based approaches, represent a promising alternative to conventional therapy.Citation2
Malignant cells of distinct histological origin, including ovarian cancer cells, can elicit tumor-specific immune responses,Citation15 suggesting that immunotherapy might be exploited to eradicate neoplastic cells from the body. However, the success of immunotherapeutic regimens is dictated by the how the tumor and the host interact in the course of therapy (e.g., whether malignant cells can establish or not immunosuppression). Importantly, some cytotoxic agents are capable of triggering an “immunogenic” cell death, wherein, chemotherapy-induced cell death invokes a tumor-specific immune response that eliminates residual tumor cells.Citation19 Anthracyclines, oxaliplatin and X-rays exemplify the stimuli that trigger bona fide immunogenic cell death.Citation19 These observations suggest that following standard surgery and chemotherapy, immunotherapy can enhance stimulate antitumor immune responses to remove the residual disease and hence to prevent relapse.Citation19 In this regard, CTAs represent a promising candidate targets for the development of anticancer immunotherapy, as they are abundantly expressed by malignant cells but virtually not by somatic tissues, exception made for the tested.Citation2 Although the biological role of CTAs is not completely understood, their association with cellular proliferation, migration and invasions is well-established.Citation2 Nevertheless, CTAs are viewed as the closest possibility to exploit the concept of the “magic bullet,” as theorized by Paul Ehrlich at the beginning of the 20th century, for cancer therapy.Citation20
The potential of specific CTAs for the implementation of antigen-specific immunotherapeutic regimens against ovarian cancer has already been proved. For instance, sperm-associated antigen 9 (SPAG9) has recently been shown to generate humoral responses in 67% of epithelial ovarian cancer patients, including in 62.5% of patients affected by early-stage (Stage I + Stage II) and in 68% of individual bearing late-stage (Stage III + Stage IV) disease.Citation3 Of note, antibodies against SPAG9 were detected in patients affected by all stages of epithelial ovarian cancer but not in healthy donors. In addition, humoral responses against SPAG9 were documented in 77% (40/52) of renal cell carcinoma,Citation4 80% (80/100) of breast cancer,Citation5 80% (54/66) of cervical cancer,Citation6 78% (92/118) of thyroid cancer,Citation7 88% (106/120) of chronic myeloid leukemia,Citation8 70% (38/54) of colorectal cancerCitation9 and 72% (36/50) of endometrial cancer patients investigated in this respect.Citation10 Owing to its immunogenicity and expression by a majority of patients, SPAG9 constitutes a promising candidate for the immunotherapeutic targeting of ovarian carcinoma. However, future studies are warranted to investigate the T-cell epitopes of SPAG9 and whether SPAG9-specific T-cell responses also develop spontaneously in cancer patients. This knowledge could be used to achieve potent tumor-specific immune responses which dendritic cell-based approaches, monoclonal antibody-based strategies and combinatorial regimens including conventional chemotherapeutics and antigen-specific immunotherapy.
Additional CTAs that have been shown to generate humoral responses in ovarian cancer patients include OY-TES-1 (10%),Citation21 LAGE-1 (25%)Citation12 and SSX (2.27%).Citation22 However, the cellular response against these antigens is yet to be explored. Recent studies indicate that malignancies are under the control of an immunosurveillance mechanism, as revealed by the direct association between the abundance of tumor-infiltrating CD8+ T lymphocytes and patient survival.Citation23 Tumor-associated antigens that have been reported to trigger a cytotoxic T-lymphocyte response include NY-ESO-1 and sperm protein 17 (SP17). The identification of a DR52b-restricted NY-ESO-1 epitope (NY-ESO-1119–143, core region 123–137) that is recognized by NY-ESO-1-specific CD4+ T-cell clones is an important step for the immunological monitoring of clinical trials targeting this important CTA.Citation24 An independent Phase I clinical trial demonstrated that NY-ESO-1 can used as a target for vaccination in advanced and high-risk ovarian cancer patients.Citation25 However, this approach is limited by absent expression of NY-ESO-1 by recurrent tumors, calling for the development of strategies that simultaneously target multiple tumor-associated antigens.Citation25 In a preclinical setting, the immunization of C57Bl/6 mice with the SP17 protein and a CpG oligonucleotide-based adjuvant protected them from the progression and spread of a syngeneic tumor.Citation13 This substantiates the possibility that SP17 may constitute a valid target for the development of anticancer vaccines. Additional CTAs that may be represent promising target for the immunotherapy of ovarian cancer are Piwil2, AKAP3 and SCP-1.Citation26-Citation28 Taken together, these observations indicate that further studies are warranted for the development of polyvalent anticancer vaccines that can counteract the multiple mechanisms of immune evasion harnessed by tumor cells.
Recently, a novel CTA, AKAP4, has been shown to be expressed and to elicit humoral immune response in multiple myeloma and prostate cancer models.Citation16,Citation17 AKAP4 was expressed in the cytoplasm and on the surface of the prostate cancer LnCaP cells as well as in tissue specimens from prostate cancer patients. In addition, an MHC-I restricted CTL response specific for AKAP4 was demonstrated in prostate cancer patients. Cytokine profiling revealed increased levels of interferon γ (IFNγ) and tumor necrosis factor α (TNFα) but no significant alterations in the levels of interleukin (IL)-4, IL-5, and IL-10, clearly indicating that a CD8+ T-cell immune response arises against AKAP4 in prostate cancer.Citation17 The ability to elicit spontaneous immune response, which are often characterized by the production of specific circulating antibodies, is a fundamental prerequisite for tumor antigens to be exploitable as targets for the immunotherapy of different tumors. In an attempt to explore the clinical implications of AKAP4 as a candidate antigen for the targeting of ovarian serous carcinoma, we analyzed the expression of AKAP4 as well as the presence of circulating anti-AKAP4 antibodies in 38 ovarian carcinoma patients. By indirect immunofluorescence assays, we detected AKAP4 at the surface of various ovarian cancer cell lines (data not shown). It is interesting to note that Stage I clinical specimens showed an elevated AKAP4 expression as compared with samples corresponding to other disease stages, suggesting that the expression of AKAP4 might be reduced along with disease progression. Formal data in support of this hypothesis, however, are missing. We observed AKAP4 expression at both the mRNA and protein levels in 86% (12/14) of early stage and 92% (22/24) of late stage ovarian carcinoma specimens but not in matched ANCTs. Histologically, AKAP4 showed a heterogeneous expression pattern. However, in most tumor specimens (94%), AKAP4+ cells accounted for more than 50% of the cell population. In addition, 65% (22/34) of patients whose ovarian cancer expressed AKAP4 exhibited an anti-AKAP4 humoral response. The elicitation of humoral responses against AKAP4 might depend on the MHC genetics of each patients, based on which patients could be classified as “responders” and “non-responders.” Importantly, the majority of our specimens corresponded to serous adenocarcinoma and serous papillary carcinoma, which expressed AKAP4 in 93% (28/30) and 100% (6/6) of cases, respectively. Moreover, 79% (19/24) serous adenocarcinoma and 67% (4/6) serous papillary carcinoma patients exhibited circulating anti-AKAP4 antibodies.
In summary, the facts that AKAP4 is expressed by malignant but not by normal cells (with the exception of testes) and that it elicits spontaneous humoral responses in patients make it an ideal candidate target for the immunotherapy of ovarian cancer. Our data demonstrate that AKAP4 is expressed by ovarian cancer cells and is highly immunogenic, laying the foundations for exploring the potential of AKAP4 as a therapeutic target in ovarian carcinoma. Future studies will have to analyze whether AKAP4 also elicits T-cell responses in ovarian cancer patients and whether AKAP4 or AKAP4-derived peptides can be successfully employed as anticancer vaccines.
Materials and Methods
Tissue specimens and serum samples
In the present study, 37 ovarian carcinoma (30 serous adenocarcinoma, 6 serous papillary carcinoma, 1 endometroid adenocarcinoma), 1 adenofibroma and 21 matched ANCTs were collected from patients attending the Safdarjung Hospital (Vardhman Mahavir Medical College; New Delhi, India) after obtaining informed written consent. This study was approved by the Institutional Human Ethical Committee of the Safdarjung Hospital as well as by the National Institute of Immunology (New Delhi, India). Tissues were fixed in RNase-free buffered formaldehyde and RNAlater for further studies. Serum samples were immediately frozen at -80°C till use.
RT-PCR studies
AKAP4 gene expression was investigated in tumor tissues and ANCT specimens by RT-PCR analysis. Briefly, tissues were homogenized and RNA was extracted using the Tri reagent (Ambion) and quantified. cDNA was synthesized using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). The following AKAP4-specific primers, which were designed from overlapping exons in order to avoid genomic DNA contamination, were used: AKAP4fwd: 5′-TGATACTACAATGATGTCTGATGAT-3′; AKAP4rev 5′GGAACTAGCAGCATCCTTGTAATCTTTATC -3′. Testis cDNA was used as a positive control. The mRNA coding for β actin was quantified as a loading control with the following primers: fwd 5′-ATCTGGCACCACACCTTCTACAATGAGCTGCG-3′; rev 5′- CGTCATACTCCTGCTTGCTGATCCACATCTGC-3′ primers. The specificity of amplicons was confirmed by sequencing.
In situ RNA hybridization
The presence of the AKAP4 mRNA was examined in serial tissue sections of ovarian cancer patients by using DIG-labeled sense and anti-sense riboprobes based on the DIG Nucleic Acid Detection Kit (Roche Diagnostics GmbH), as previously described.Citation14
Immunohistochemical analyses
Serial sections of ovarian carcinoma specimens were subjected to deparaffinization and rehydration in a series of alcohol. Sections were treated with 10% hydrogen peroxide in methanol for 1 h to quench endogenous peroxidases. The blocking of unspecific binding sites was achieved by incubating samples in 5% goat serum for 1 h at room temperature (RT). Subsequently, sections were probed with rat polyclonal anti-AKAP4 antibodies at 4°C overnight (in a humidified chamber). Primary antibodies were revealed with horseradish peroxidase-conjugated goat anti-rat IgGs (Jackson ImmunoResearch Laboratories) and 3,3′-diaminobenzidine (Sigma-Aldrich) as a substrate. Sections were counterstained with hematoxylin and eosin, mounted and imaged. The AKAP4 immunoreactivity score (AKAP4+ cells) was calculated by a senior pathologist as the percentage of AKAP4 immunoreactive cells among 500 cells from 5 random fields. Specimens exhibiting ≤ 10% AKAP4+ cells were considered negative for AKAP4 expression.
ELISA tests
Humoral responses against recombinant AKAP4 in the serum of ovarian carcinoma patients sera were analyzed by ELISA. Plates were coated with 500 ng recombinant purified AKAP4 and incubated at 4°C overnight. Non-specific binding sites were blocked by incubating plates with 3% non-fat skimmed milk for 1 h at RT. Plates were then incubated with (1:100 diluted) sera from ovarian cancer patients and healthy donors for 2 h at RT and subsequently with an anti-human IgG antibody (Jackson ImmunoResearch Laboratories) for 1 h at RT. o-phenylenediamine dihydrochloride was employed as a substrate and the colorimetric reaction was stopped with 5N H2SO4. The mean + 2SD value of the antibody titer of healthy donors was used as a cut-off value, above which all samples were considered positive. The specificity of circulating antibodies was confirmed by immunoblotting, as previously described.Citation10 In addition, neutralization experiments were performed by pre-incubating sera with 15 μg/mL recombinant AKAP4 for 2 h at RT. Thereafter, neutralized sera were used for probing recombinant AKAP4 by immunoblotting, following standardized procedures.
Statistical analyses
Statistical analyses were performed by using the SPSS 20.0 software. p values < 0.05 were considered as statistically significant.
| Abbreviations: | ||
| AKAP4 | = | A-kinase anchor protein 4 |
| ANCT | = | adjacent non-cancerous tissue |
| CTA | = | cancer-testis antigen |
| SP17 | = | sperm protein 17 |
| SPAG9 | = | sperm associated antigen 9 |
Acknowledgments
This work is supported by grants from Indo-UK Cancer Research Program, Centre for Molecular Medicine, NII-core funding, Department of Biotechnology, and the government of India.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Notes
† These authors contributed equally to this work.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62:10 - 29; http://dx.doi.org/10.3322/caac.20138; PMID: 22237781
- Suri A, Saini S, Sinha A, Agarwal S, Verma A, Parashar D, et al. Cancer testis antigens: A new paradigm for cancer therapy. Oncoimmunology 2012; 1:1194 - 6; http://dx.doi.org/10.4161/onci.20686; PMID: 23170277
- Garg M, Chaurasiya D, Rana R, Jagadish N, Kanojia D, Dudha N, et al. Sperm-associated antigen 9, a novel cancer testis antigen, is a potential target for immunotherapy in epithelial ovarian cancer. Clin Cancer Res 2007; 13:1421 - 8; http://dx.doi.org/10.1158/1078-0432.CCR-06-2340; PMID: 17332284
- Garg M, Kanojia D, Khosla A, Dudha N, Sati S, Chaurasiya D, et al. Sperm-associated antigen 9 is associated with tumor growth, migration, and invasion in renal cell carcinoma. Cancer Res 2008; 68:8240 - 8; http://dx.doi.org/10.1158/0008-5472.CAN-08-1708; PMID: 18922895
- Kanojia D, Garg M, Gupta S, Gupta A, Suri A. Sperm-associated antigen 9, a novel biomarker for early detection of breast cancer. Cancer Epidemiol Biomarkers Prev 2009; 18:630 - 9; http://dx.doi.org/10.1158/1055-9965.EPI-08-0629; PMID: 19190149
- Garg M, Kanojia D, Salhan S, Suri S, Gupta A, Lohiya NK, et al. Sperm-associated antigen 9 is a biomarker for early cervical carcinoma. Cancer 2009; 115:2671 - 83; http://dx.doi.org/10.1002/cncr.24293; PMID: 19326449
- Garg M, Kanojia D, Suri S, Gupta S, Gupta A, Suri A. Sperm-associated antigen 9: a novel diagnostic marker for thyroid cancer. J Clin Endocrinol Metab 2009; 94:4613 - 8; http://dx.doi.org/10.1210/jc.2009-0703; PMID: 19820019
- Kanojia D, Garg M, Saini S, Agarwal S, Kumar R, Suri A. Sperm associated antigen 9 expression and humoral response in chronic myeloid leukemia. Leuk Res 2010; 34:858 - 63; http://dx.doi.org/10.1016/j.leukres.2010.01.017; PMID: 20138665
- Kanojia D, Garg M, Gupta S, Gupta A, Suri A. Sperm-associated antigen 9 is a novel biomarker for colorectal cancer and is involved in tumor growth and tumorigenicity. Am J Pathol 2011; 178:1009 - 20; http://dx.doi.org/10.1016/j.ajpath.2010.11.047; PMID: 21356354
- Yu P, Yan L, Zhang H, Lin X, Zhao X. Expression and clinical significance of sperm-associated antigen 9 in patients with endometrial carcinoma. Int J Gynecol Cancer 2012; 22:87 - 93; http://dx.doi.org/10.1097/IGC.0b013e3182370f2e; PMID: 22146769
- Ghafouri-Fard S, Modarressi MH. Cancer-testis antigens: potential targets for cancer immunotherapy. Arch Iran Med 2009; 12:395 - 404; PMID: 19566358
- Odunsi K, Jungbluth AA, Stockert E, Qian F, Gnjatic S, Tammela J, et al. NY-ESO-1 and LAGE-1 cancer-testis antigens are potential targets for immunotherapy in epithelial ovarian cancer. Cancer Res 2003; 63:6076 - 83; PMID: 14522938
- Chiriva-Internati M, Yu Y, Mirandola L, Jenkins MR, Chapman C, Cannon M, et al. Cancer testis antigen vaccination affords long-term protection in a murine model of ovarian cancer. PLoS One 2010; 5:e10471; http://dx.doi.org/10.1371/journal.pone.0010471; PMID: 20485677
- Mohapatra B, Verma S, Shankar S, Suri A. Molecular cloning of human testis mRNA specifically expressed in haploid germ cells, having structural homology with the A-kinase anchoring proteins. Biochem Biophys Res Commun 1998; 244:540 - 5; http://dx.doi.org/10.1006/bbrc.1998.8079; PMID: 9514854
- Vermeij R, Daemen T, de Bock GH, de Graeff P, Leffers N, Lambeck A, et al. Potential target antigens for a universal vaccine in epithelial ovarian cancer. Clin Dev Immunol 2010; 2010:1505 - 15; http://dx.doi.org/10.1155/2010/891505; PMID: 20885926
- Chiriva-Internati M, Raffaele F, Yu Y, Hamrick C, Gagliano N, Grizzi F, et al. AKAP4: a novel cancer testis antigen for multiple myeloma. Br J Haematol 2008; 140:465 - 68; http://dx.doi.org/10.1111/j.1365-2141.2007.06940.x; PMID: 18162122
- Chiriva-Internati M, Yu Y, Mirandola L, D’Cunha N, Hardwicke F, Cannon MJ, et al. Identification of AKAP-4 as a new cancer/testis antigen for detection and immunotherapy of prostate cancer. Prostate 2012; 72:12 - 23; http://dx.doi.org/10.1002/pros.21400; PMID: 21520158
- Taylor SE, Kirwan JM. Ovarian cancer: current management and future directions. Obstetrics, Gynaecol Reprod Med 2012; 22:33 - 7; http://dx.doi.org/10.1016/j.ogrm.2011.11.003
- Kepp O, Tesniere A, Zitvogel L, Kroemer G. The immunogenicity of tumor cell death. Curr Opin Oncol 2009; 21:71 - 6; http://dx.doi.org/10.1097/CCO.0b013e32831bc375; PMID: 19125021
- Scudellari M. A ballsy search for cancer targets. Nat Med 2011; 17:916 - 8; http://dx.doi.org/10.1038/nm0811-916; PMID: 21818078
- Tammela J, Uenaka A, Ono T, Noguchi Y, Jungbluth AA, Mhawech-Fauceglia P, et al. OY-TES-1 expression and serum immunoreactivity in epithelial ovarian cancer. Int J Oncol 2006; 29:903 - 10; PMID: 16964386
- Valmori D, Qian F, Ayyoub M, Renner C, Merlo A, Gnjatic S, et al. Expression of synovial sarcoma X (SSX) antigens in epithelial ovarian cancer and identification of SSX-4 epitopes recognized by CD4+ T cells. Clin Cancer Res 2006; 12:398 - 404; http://dx.doi.org/10.1158/1078-0432.CCR-05-1902; PMID: 16428478
- Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A 2005; 102:18538 - 43; http://dx.doi.org/10.1073/pnas.0509182102; PMID: 16344461
- Bioley G, Dousset C, Yeh A, Dupont B, Bhardwaj N, Mears G, et al. Vaccination with recombinant NY-ESO-1 protein elicits immunodominant HLA-DR52b-restricted CD4+ T cell responses with a conserved T cell receptor repertoire. Clin Cancer Res 2009; 15:4467 - 74; http://dx.doi.org/10.1158/1078-0432.CCR-09-0582; PMID: 19531622
- Diefenbach CS, Gnjatic S, Sabbatini P, Aghajanian C, Hensley ML, Spriggs DR, et al. Safety and immunogenicity study of NY-ESO-1b peptide and montanide ISA-51 vaccination of patients with epithelial ovarian cancer in high-risk first remission. Clin Cancer Res 2008; 14:2740 - 8; http://dx.doi.org/10.1158/1078-0432.CCR-07-4619; PMID: 18451240
- Lee JH, Schütte D, Wulf G, Füzesi L, Radzun HJ, Schweyer S, et al. Stem-cell protein Piwil2 is widely expressed in tumors and inhibits apoptosis through activation of Stat3/Bcl-XL pathway. Hum Mol Genet 2006; 15:201 - 11; http://dx.doi.org/10.1093/hmg/ddi430; PMID: 16377660
- Sharma S, Qian F, Keitz B, Driscoll D, Scanlan MJ, Skipper J, et al. A-kinase anchoring protein 3 messenger RNA expression correlates with poor prognosis in epithelial ovarian cancer. Gynecol Oncol 2005; 99:183 - 8; http://dx.doi.org/10.1016/j.ygyno.2005.06.006; PMID: 16005946
- Tammela J, Jungbluth AA, Qian F, Santiago D, Scanlan MJ, Keitz B, et al. SCP-1 cancer/testis antigen is a prognostic indicator and a candidate target for immunotherapy in epithelial ovarian cancer.. Cancer Immun 2004; 4:10; PMID: 15487888