Abstract
Recently, a number of studies have documented the presence of high endothelial venules in both mouse and human tumors. The significance of these highly specialized blood vessels within neoplastic lesions, and notably their capacity to influence anticancer immune responses and tumor progression, remains to be fully understood.
Using a well-established mouse model of chemical carcinogenesis (methylcholanthrene, MCA),Citation1 we have recently demonstrated that the specific ablation of Foxp3+ regulatory T cells (Tregs) can result in markedly reduced tumor growth rates and even tumor regression (in some animals).Citation2 Unexpectedly, the ability of Treg-depleted mice to control disease progression was not associated with the degree of immune activation but with the extent of intratumoral T-cell infiltration. Moreover, we observed an absolute correlation between the levels of tumor-infiltrating T cells and presence within neoplastic lesions of specialized blood vessels, namely high endothelial venules (HEVs). What is therefore the significance of intratumoral HEVs? These vessels, which are normally found in secondary lymphoid organs, allow naïve T cells and central memory cells to enter lymph nodes upon interactions between CD62L and ligands expressed on HEV. HEVs have also been observed at sites of chronic inflammation and in autoimmune lesions (reviewed in ref. Citation3). It is indeed possible that HEVs contribute to the pathogenesis of these diseases by sustaining the accumulation of T cells at the diseased site, hence contributing to the chronicity of the immune response and promoting tissue damage. Nevertheless, HEVs may be useful in tumors as the sustained ingress of T cells into the neoplastic lesion may promote effective antitumor immune responses. This hypothesis is supported by our own findings in mice as well as by recent reports indicating that intratumoral HEVs are associated with a good prognosis in breast cancer patients.Citation4
What is the significance of our findings, originating from a mouse model of MCA-induced carcinogenesis? First, we observed HEVs within neoplastic lesions only after the depletion of Tregs. Thus, at least in this model, the removal of Tregs is an essential prerequisite to the development of HEVs.Citation2 These data point to a potential link between Tregs and the differentiation of blood vessels. Perhaps, Tregs prevent the neogenesis of HEVs in conditions of chronic inflammation by limiting dendritic cell activation and/or the production of pro-inflammatory cytokines such as lymphotoxin αβ (ΛΤαβ).
Second, we only observed HEVs within the neoplastic lesions of a fraction of Treg-depleted animals. This finding indicates that while the depletion of Treg may be necessary for HEV development, it is certainly not sufficient.Citation2 Why HEVs develop in the tumors of some, but not all, Treg-depleted mice remains to be clarified. The mechanism(s) that account for this observation may be linked to the nature of the immune response that develops upon the depletion of Tregs. For instance, the T cells and/or B cells of some, but not all, mice might produce LTαβ or other important HEV-inducing factors.Citation5 Alternatively, the development of HEV may be dictated by the nature of developing tumor. For instance, the tumor might specifically impinge on the immune response or it might produce factors that favor or inhibit HEV formation. The identification of the mechanisms that underlie the development of intratumoral HEVs may provide valuable clues for harnessing HEV to boost anticancer immune responses in several settings.
Our studies as well as reports describing the prognostic significance of HEVs in melanoma and breast cancer patients ascribe beneficial effects to intratumoral HEVs. A note of caution, however, comes from an elegant report by Shields and colleagues. By means of tumor cell lines engineered to produce varying levels of the chemokine CCL21, the authors of this study demonstrated that the development of lymphoid-like features (including HEVs) within tumors conferred on them the ability to inhibit anticancer immune responses by enabling the entry of Tregs and myeloid derived suppressor cells (MDSCs).Citation6 These data are particularly interesting in light of a previous study by Schneider and colleagues, who examined the impact of CCR7 deficiency on the development of adaptive immune responses. Surprisingly, the authors found that antigen-specific T-cell responses were delayed but actually increased in Ccr7−/− mice, as compared with their wild-type littermates.Citation7 Moreover, the major impact of CCR7 deficiency reflected an impaired immune regulation stemming from a dramatic decrease in the abundance of Tregs within lymph nodes of Ccr7−/− mice. These data point to a tolerogenic role for CCR7 and its ligands, CCL19 and CCL21, and explain why the intratumoral expression of these chemokines might facilitate immunosuppression, as suggested by Shields and colleagues. Thus, the presence of HEVs may not always be indicative of a microenvironment that supports productive antitumor immune responses.
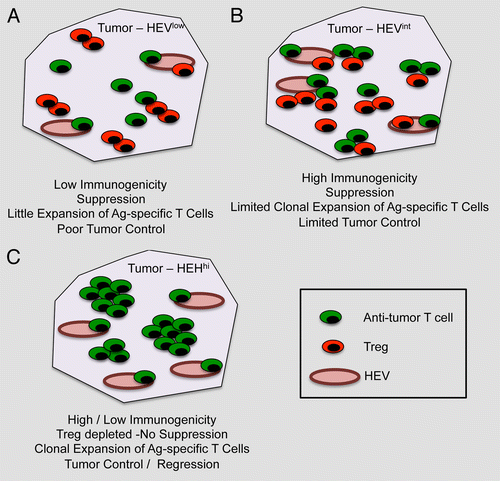
Collectively, the results of these studies suggest that simply inducing HEVs in tumors is not sufficient to activate antitumor immune responses. These will indeed depend upon the inherent immunogenicity of malignant cells as well as upon the presence of local signals for the priming of antigen-specific T cells. Hypothetically, in the case of a poorly immunogenic tumor, HEVs may promote the recruitment of both conventional T cells and Tregs, which would presumably result in a limited expansion of tumor-specific T cells and no control of tumor progression (). Conversely, in the case of highly immunogenic tumors, the expansion of tumor-specific T cells may occur in spite of the concomitant ingress of both Tregs and conventional T cell via HEVs, resulting in a moderate control of tumor progression (). Our study suggests that Treg depletion lowers the threshold for both immune cell activation and HEV neogenesis, the overall effect of which is the elicitation of robust antitumor T-cell responses, perhaps even when tumors are of low immunogenicity (). This results in a significant control of tumor progression and, at least in some cases, tumor regression. Should this model be confirmed by independent studies, combining Treg-depleting regimens with strategies for the intratumoral neogenesis of HEVs may turn out to constitute a highly effective form of cancer immunotherapy.
Figure 1. Impact of high endothelial venules on tumor progression. (A) In the case of poorly immunogenic tumors, high endothelial venules (HEVs) are not abundant (HEVlow) and the neoplastic lesion is infiltrated by both conventional T cells and Foxp3+ regulatory T cells (Tregs). In this setting, antitumor T cells have limited chances to expand and tumor growth remains virtually unaffected.(B) When tumor are highly immunogenic, the number of intratumoral HEVs is slightly increased (HEVint), resulting in a relatively more intense recruitment of conventional T cells and Tregs. In this case, tumor-specific T cells may undergo some expansion, yet the presence of Tregs limits their antineoplastic effects. (C) The depletion of Tregs not only limits immunosuppression but also greatly increase the number of intratumoral HEVs (HEVhi). This results in the elicitation of robust antitumor immune responses that can control disease progression (at least theoretically) irrespective of the immunogenicity of malignant cells.
References
- Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol 2011; 29:235 - 71; http://dx.doi.org/10.1146/annurev-immunol-031210-101324; PMID: 21219185
- Hindley JP, Jones E, Smart K, Bridgeman H, Lauder SN, Ondondo B, et al. T-cell trafficking facilitated by high endothelial venules is required for tumor control after regulatory T-cell depletion. Cancer Res 2012; 72:5473 - 82; http://dx.doi.org/10.1158/0008-5472.CAN-12-1912; PMID: 22962270
- Drayton DL, Liao S, Mounzer RH, Ruddle NH. Lymphoid organ development: from ontogeny to neogenesis. Nat Immunol 2006; 7:344 - 53; http://dx.doi.org/10.1038/ni1330; PMID: 16550197
- Martinet L, Garrido I, Filleron T, Le Guellec S, Bellard E, Fournie JJ, et al. Human solid tumors contain high endothelial venules: association with T- and B-lymphocyte infiltration and favorable prognosis in breast cancer. Cancer Res 2011; 71:5678 - 87; http://dx.doi.org/10.1158/0008-5472.CAN-11-0431; PMID: 21846823
- Moussion C, Girard JP. Dendritic cells control lymphocyte entry to lymph nodes through high endothelial venules. Nature 2011; 479:542 - 6; http://dx.doi.org/10.1038/nature10540; PMID: 22080953
- Shields JD, Kourtis IC, Tomei AA, Roberts JM, Swartz MA. Induction of lymphoidlike stroma and immune escape by tumors that express the chemokine CCL21. Science 2010; 328:749 - 52; http://dx.doi.org/10.1126/science.1185837; PMID: 20339029
- Schneider MA, Meingassner JG, Lipp M, Moore HD, Rot A. CCR7 is required for the in vivo function of CD4+ CD25+ regulatory T cells. J Exp Med 2007; 204:735 - 45; http://dx.doi.org/10.1084/jem.20061405; PMID: 17371928