Abstract
The combination of BRAF-targeted agents with immune checkpoint inhibitors represents a recent advance in the treatment of melanoma, even though each of these therapeutic approaches alone has specific limitations. Increasing evidence suggests indeed the existence of a synergistic interaction between these therapeutic modalities.
Keywords: :
During the past decade, key mutations have been identified that contribute to the development and malignant progression of melanoma, hence standing out as potential targets for therapeutic interventions. Mutations in v-raf murine sarcoma viral oncogene homolog B1 (BRAF) occur in about half of melanomas and generally lead to an activation of the mitogen-activated protein kinase (MAPK) signaling pathway. In turn, this promotes oncogenesis and tumor progression through several mechanisms, including accrued proliferation rates, resistance to apoptotic stimuli as well as increased angiogenic, invasive and metastatic potential.
The treatment of BRAF-mutated melanomas with agents that specifically target this oncogenic alteration represents one of the most significant therapeutic advances in this setting over decades. Results from a Phase III clinical trial testing vemurafenib (an inhibitor of BRAF) vs. standard of care, dacarbazine-based chemotherapy demonstrated that the former can significantly improve progression-free and overall survival,Citation1 leading to its approval by FDA in 2011. However, clinical responses to vemurafenib are generally temporary, with a median time to progression of only 5.3 mon.Citation1
Intense efforts are currently being dedicated at the identification of strategies that may prolong clinical responses to vemurafenib. To this aim, BRAF-targeted agents have been tested in combination with other treatment modalities. One of these strategies involved the concurrent administration of BRAF and MAPK/ERK kinase (MEK) inhibitors, de facto targeting two distinct nodes of the same signaling pathway. This combinatorial regimen appears to indeed extend progression-free survival, yet the majority of patients progress on therapy within 10 mon.Citation2 Alternative strategies are therefore needed to produce long-lasting clinical responses.
Another successful approach to the treatment of melanoma involves immunotherapy. In particular, the use of immune checkpoint inhibitors has shown tremendous promise, leading to the approval of ipilimumab, a monoclonal antibody that blocks the immunomodulatory molecule cytotoxic T-lymphocyte-associated protein 4 (CTLA4), by FDA in 2011.Citation3 Additional checkpoint inhibitors that are currently being tested in clinical trials include monoclonal antibodies that specifically block programmed cell death 1 (PDCD1, best known as PD1) or it main ligand CD274 (best known as PDL1).Citation4,Citation5 The administration of immunotherapy to melanoma patients as a standalone intervention may result in long-term responses, though the overall response rate is relatively low (15% upon CTLA4 blockade, 40% upon PD1 blockade).Citation3,Citation4
Mounting evidence indicates that oncogenic BRAF signaling contributes to immune escape and that targeting BRAF mutations may increase the immunogenicity of melanoma cells.Citation6 This has significant translational implications and provides the rationale for combining BRAF-targeting agents with immunotherapy for the treatment of melanoma. The first experimental evidence in support of this notion was published in 2010. In particular, it was shown that the administration of BRAF inhibitors to melanoma cell lines and fresh melanoma digests in vitro induces the upregulation of melanoma differentiation antigens.Citation6 Importantly, such an increase in the antigenic properties of melanoma cells was associated with an enhanced recognition by antigen-specific T cells.Citation6 Interestingly, MEK inhibitors were found to have a deleterious effect on T-cell function, whereas BRAF inhibitors were not. Following this influential discovery, we and others analyzed several immunological parameters in metastatic melanoma patients receiving BRAF inhibitors, finding a dramatic increase in tumor-infiltrating CD8+ T cells in patients within 10–14 d of BRAF-targeted therapy initiation.Citation7,Citation8
The immunological effects of BRAF inhibition have recently been further characterized. In particular, BRAF-targeted agents have been associated with the establishment of a therapeutically favorable tumor microenvironment. Indeed, along with a robust accumulation of tumor-infiltrating CD8+ T cells, the expression of melanoma-differentiation antigens and T-cell activation markers were both increased. Conversely, of the levels of immunosuppressive cytokines such as interleukin (IL)-6 and IL-8 were decreased.Citation8 This is consistent with recent studies demonstrating a decrease in the production of IL-1α by tumor-associated fibroblasts as well as a decrease in stromal vascular endothelial growth factor (VEGF) expression upon BRAF inhibition.Citation9,Citation10 Of note, both the expression of melanoma-differentiation antigens and the CD8+ T-cell infiltrate were found to decrease again at the time of disease progression.
Importantly, BRAF inhibition was associated with an increased expression of the immunosuppressive molecules PD1 and hepatitis A virus cellular receptor 2 (HAVCR2, best known as TIM3) on T cells, as well as of the immunosuppressive ligand PDL1 on tumor cells, within 10–14 d of treatment initiation.Citation8 The presence of PD1 and TIM3 on T cells is likely to reflect their activation status. Conversely, such an early increase in the expression of PDL1 on the tumor cells was completely unexpected. This constellation of findings has important implications, as the T cells that are infiltrating these tumors may be inhibited by PDL1, potentially blunting the immune response early in the course of therapy. Moreover, it suggests that the use of immune checkpoint inhibitors (such as anti-CTLA4, anti-PD1 or anti-PDL1 antibodies) together with BRAF-targeting agents may significantly increase their therapeutic potential ().
Figure 1. Oncogenic BRAF contributes to immune escape through the downregulation of melanoma-differentiation antigens and by establishing an immunosuppressive tumor microenvironment. The administration of a BRAF inhibitor promotes clinical responses along with an increased expression of melanoma-differentiation antigens by malignant cells, an increased tumor infiltration by CD8+ T cells, and a decreased production of immunosuppressive cytokines such as interleukin (IL) -6, IL-8 and IL-1α as well as of the angiogenic mediator vascular endothelial growth factor (VEGF). This phenotype is reverted at time of disease progression. Importantly, the expression of immunomodulatory molecules on T cells (e.g., PD1) and on tumor cells (e.g., PDL1) is also increased within 14 d of BRAF-targeted therapy initiation. Taken together, these data suggest that the therapeutic potential of BRAF-targeted agents may be significantly improve by the early blockade of immune checkpoints.
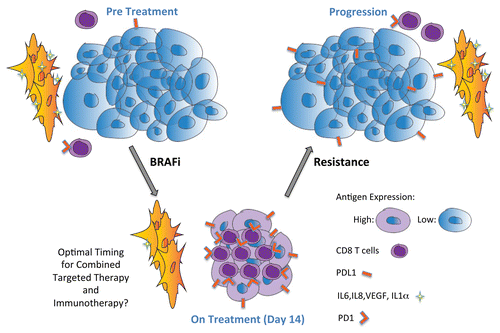
Clinical trials testing the combination of BRAF-targeting agents and immunotherapy are currently underway. The sequence and timing of this combination therapy deserves an attentive consideration. Current data suggest indeed that (1) BRAF-targeted therapy should be initiated first, to enhance antigen expression by malignant cells and allow for tumor infiltration by CD8+ T cells, and (2) that checkpoint inhibitors should be given early in the course of BRAF-targeted therapy rather than at the time of progression.
However, several questions remain have not yet been answered. For instance, will the combination of BRAF inhibitors and immune checkpoint blockers increase the duration of clinical responses as well as their rate? Will this result in an increased rate of adverse effects? What are the appropriate timing, sequence, and duration of this therapy? Can similar effects be obtained with MEK inhibitors or with combinatorial regimens involving BRAF and MEK inhibitors? And finally, can we translate this strategy to other malignancies? Answering these questions require thoughtful correlative studies in the context of appropriately designed clinical trials as well as in preclinical settings, based on genetically-engineered mouse models. These studies are currently underway, and the results will surely guide the rational combination of BRAF-targeted agents and immunotherapy for the treatment of melanoma.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al, BRIM-3 Study Group. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011; 364:2507 - 16; http://dx.doi.org/10.1056/NEJMoa1103782; PMID: 21639808
- Flaherty KT, Infante JR, Daud A, Gonzalez R, Kefford RF, Sosman J, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med 2012; 367:1694 - 703; http://dx.doi.org/10.1056/NEJMoa1210093; PMID: 23020132
- Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711 - 23; http://dx.doi.org/10.1056/NEJMoa1003466; PMID: 20525992
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012; 366:2443 - 54; http://dx.doi.org/10.1056/NEJMoa1200690; PMID: 22658127
- Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012; 366:2455 - 65; http://dx.doi.org/10.1056/NEJMoa1200694; PMID: 22658128
- Boni A, Cogdill AP, Dang P, Udayakumar D, Njauw CN, Sloss CM, et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res 2010; 70:5213 - 9; http://dx.doi.org/10.1158/0008-5472.CAN-10-0118; PMID: 20551059
- Wilmott JS, Long GV, Howle JR, Haydu LE, Sharma RN, Thompson JF, et al. Selective BRAF inhibitors induce marked T-cell infiltration into human metastatic melanoma. Clin Cancer Res 2012; 18:1386 - 94; http://dx.doi.org/10.1158/1078-0432.CCR-11-2479; PMID: 22156613
- Frederick DT, Piris A, Cogdill AP, Cooper ZA, Lezcano C, Ferrone CR, et al. BRAF Inhibition Is Associated with Enhanced Melanoma Antigen Expression and a More Favorable Tumor Microenvironment in Patients with Metastatic Melanoma. Clin Cancer Res 2013; 19:1225 - 31; http://dx.doi.org/10.1158/1078-0432.CCR-12-1630; PMID: 23307859
- Khalili JS, Liu S, Rodríguez-Cruz TG, Whittington M, Wardell S, Liu C, et al. Oncogenic BRAF(V600E) promotes stromal cell-mediated immunosuppression via induction of interleukin-1 in melanoma. Clin Cancer Res 2012; 18:5329 - 40; http://dx.doi.org/10.1158/1078-0432.CCR-12-1632; PMID: 22850568
- Liu C, Peng W, Xu C, Lou Y, Zhang M, Wargo JA, et al. BRAF inhibition increases tumor infiltration by T cells and enhances the antitumor activity of adoptive immunotherapy in mice. Clin Cancer Res 2013; 19:393 - 403; http://dx.doi.org/10.1158/1078-0432.CCR-12-1626; PMID: 23204132