Abstract
Metastatic melanoma is near-to-invariably a fatal disease. As novel therapeutic strategies against metastatic melanoma are urgently needed, we have tested a combinatorial regimen consisting of conventional chemotherapy coupled to bevacizumab, a monoclonal antibody that inhibit angiogenesis, demonstrating some clinical benefit. A preliminary assessment of one of our clinical trials points to a previously unrecognized immunomodulatory effect of bevacizumab. Herein, we evaluate the immunomodulatory effect of bevacizumab when administered together with conventional chemotherapy to patients with metastatic melanoma. To this aim, we measured the abundance of various lymphocyte subsets among peripheral blood mononuclear cells (PBMCs) as well as the circulating levels of 42 cytokines, chemokines and growth factors in patients with metastatic melanoma who received albumin-bound paclitaxel plus carboplatin, either as a standalone intervention (AC, 55 subjects) or combined with bevacizumab (ACB, 39 individuals), in the context of clinical trials N057e and N0775, respectively. Relative shifts in PBMC subsets and cytokine levels were calculated (relative to baseline levels) when patients underwent restaging evaluation after two cycles of therapy. The Mann–Whitney U test was used to compare responses between the groups. Bevacizumab failed to affect the TH1/TH2 cell ratio in this patient cohort. However, we observed a significant increase in CD8+ lymphocytes in patients who received ACB (+38%) but not in subjects treated with AC only (−10%) (p = 0.03). Moreover, circulating interleuikin-6 (IL-6) levels were reduced in patients treated with ACB (−42%) but not in individuals receiving AC only (28%) (p = 0.0018). Thus, the addition of bevacizumab to chemotherapy for the treatment of metastatic melanoma exerts immunomodulatory effects.
Introduction
Melanoma is the fifth and sixth most common malignancy in men and women, respectively, in the United States.Citation1 Although patients with early stage disease can be cured with resection, the prognosis of individuals affected by metastatic melanoma is dismal.Citation2-Citation4 The recent approval of two novel agents for the treatment of metastatic melanoma is encouraging and offer opportunities for the development of innovative combinatorial regimens taking advantage of complementary aspects of different therapeutics (e.g., BRAF inhibitors, immunomodulating agents, conventional chemotherapies).Citation5-Citation7
Over the past several years we have explored the therapeutic potential of combining conventional cytotoxic therapy with inhibitors of angiogenesis for the treatment of patients affected by metastatic melanoma. In a series of exploratory multicenter Phase 2 clinical trials, we have demonstrated that adding bevacizumab, an anti-angiogenic monoclonal antibody, to taxane-based chemotherapy may provide clinical benefits to this patient population. The combination of carboplatin and paclitaxel has been shown to exert antineoplastic effect in patients with metastatic melanoma.Citation8 Albumin-bound paclitaxel, ABI-007, has been developed to reduce the toxicity of the previous formulation involving the Cremophor® vehicle. We examined the activity of ABI-007 coupled to carboplatin in two separate clinical trials. In one of these Phase II studies, N057e, the combination of ABI-007 and carboplatin (AC) was associated with an overall survival of 11.1 mo in chemotherapy-naïve patients, and of 10.9 mo in previously treated patients.Citation9 In another Phase II trial, N0775, we combined bevacizumab with ABI-007 and carboplatin (ACB). The median overall survival of patients who received ACB in this study was 15.4 months.Citation10
Bevacizumab is a recombinant chimeric monoclonal antibody targeting the vascular endothelial growth factor (VEGF) and is currently used as an angiogenesis inhibitor in subjects affected by a broad range of malignancies.Citation11-Citation13 Although controversial, there is evidence that bevacizumab may add benefit to conventional chemotherapy in melanoma patients.Citation14 We have recently demonstrated that VEGF mediates a shift in the polarization of helper T lymphocytes toward a state of “chronic inflammation” featuring a TH2 bias that is commonly observed in untreated patients with metastatic melanoma.Citation15 Additionally, VEGF has been found to inhibit both the development of dendritic cells (DCs)Citation16 and mature DC function.Citation17 Hence, we decided to investigate systemic immunity in patients with metastatic melanoma treated with AC-based chemotherapy alone or combined with bevacizumab, in order to determine whether bevacizumab can reverse the immunosuppressive effects of VEGF in this setting.
Results
Changes in peripheral blood mononuclear cell subsets in response to AC- vs. ACB-based chemotherapy
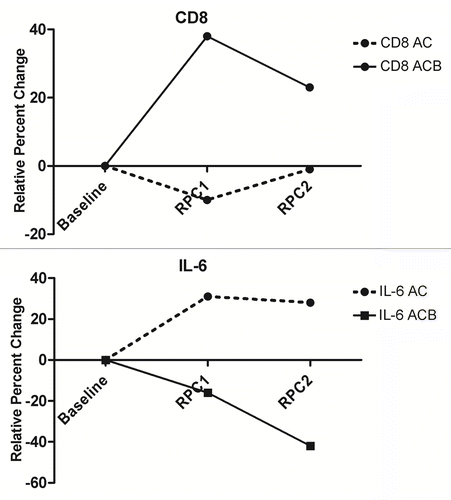
We compared the changes in the relative abundance of CD3+, CD4+, CD8+, CD3+CD62L+, CD4+TIM3+ and CD4+CD294+ cells among the peripheral blood mononuclear cells (PBMCs) of patients with metastatic melanoma before and after AC-based chemotherapy alone or combined with bevacizumab (ACB). After one cycle of therapy, we observed a significant increase of circulating CD8+ cells in patients receiving ACB (+38%, −21% to 72%) (median, interquartile range) but not in subjects who were treated with AC alone (−10%, −32% to 42%) (p = 0.03) (; ). Patients receiving AC only exhibited 5.4% (2.7% to 11.7%) CD8+ cells at baseline, 4.4% (2.4% to 11.0%) after one cycle of treatment and 5.0% (2.5% to 9.6%) after two cycles. Conversely, individuals treated with ACB exhibited 11.0% (6.3% to 18.1%) CD8+ cells at baseline, 11.0% (7.7% to 20.1%) after one cycle of treatment and 11.9% (7.3% to 18.8%) after two. We detected no significant differences in other PBMC subsets after the first cycle of therapy. In addition, we found no significant differences in all PBMC subsets after the second cycle of therapy. The median TH1/TH2 (CD4+TIM3+/CD4+CD294+) cell ratio of all patients in this study was 0.76. After two cycles of treatment, patients who received bevacizumab together with chemotherapy manifested a 5% (−24% to 39%) increase in the TH1/TH2 cell ratio, while subjects treated with chemotherapy alone exhibited a −4% (−25% to 28%) shift (p = 0.28) in this ratio. The relative changes in the abundance of lymphocyte subsets prior to the second cycle of therapy were not associated with progression-free survival (data not shown).
Table 1. PBMC phenotypes*
Figure 1. Changes in circulating CD8+ lymphocytes and interleukin-6 levels in melanoma patients receiving albumin-bound paclitaxel plus carboplatin alone or combined with bevacizumab. The median relative percent changes (RPCs) of CD8+ lymphocytes and circulating interleukin-6 (IL-6) levels are displayed for patients who received one (RPC1) or two (RPC2) cycles of chemotherapy alone (AC) or combined with bevacizumab (ACB). For CD8+ lymphocytes, the difference between patients receiving ACB and AB was significant after one (p = 0.03), but not after two (p = 0.38), cycles of therapy. For IL-6, the difference was significant after one (p = 0.01) as well as after two (p = 0.0018) cycles of therapy.
Changes in circulating cytokines, chemokines and growth factors in response to AC- vs. ACB-based chemotherapy
After one cycle of treatment, we observed a significant decrease in the circulating levels of interleukin-6 (IL-6; −16%, −63% to 38%) in patients who received bevacizumab together with chemotherapy but not in individuals treated with chemotherapy alone (31%, −36% to 527%) (p = 0.02) (; ). After the second cycle of therapy, the levels of circulating IL-6 decreased further in patients treated with ACB (−42%, −88 to 0%) but not in subjects who received AC only (28%, −37% to 480%) (p = 0.0018) (Table S1). We also observed a significant decrease in the circulating levels of IFNγ-inducible protein 10 (IP-10) in patients receiving ACB but not in individuals treated with AB only, after the second cycle of therapy (). We do not believe that the differences in circulating tumor necrosis factor α (TNFα) levels were significant as the shift of the median value was within the coefficient of variation of the test. Along similar lines, the levels of several other cytokines, chemokines and growth factors did not differ in patients receiving one or two cycles of AB- or ACB-based chemotherapy (). Although after two cycles of therapy we observed a greater decrease in the circulating levels of VEGF among patients who received ACB (−49%, −79% to −11%) than among those treated with AC only (−27%, −56% to −7%), this difference was not statistically significant (p = 0.09). The relative changes in the circulating levels of all cytokines, chemokines and growth factors tested prior to the second cycle of therapy were not associated with progression free-survival (data not shown).
Table 2. Cytokines, chemokines and growth factors
Discussion
Bevacizumab, a drug designed for and best known for its anti-angiogenic effects, exerts immunomodulatory effects when administered in combination with chemotherapy to subjects affected by metastatic melanoma. Patients who received one cycle of bevacizumab together with albumin-bound paclitaxel and carboplatin (ACB) demonstrated an increase in CD8+ lymphocytes that persisted after the second cycle of treatment. Conversely, we observed a significant decrease in the circulating levels of IL-6 after each cycle of ACB-based chemotherapy. Bevacizumab-receiving patients also manifested a greater reduction in circulating VEGF levels and an increase in the TH1/TH2 cell ratio than subjects who received chemotherapy alone, though these changes were not significant.
Increased amounts of circulating CD8+ lymphocytes suggest that more cytotoxic T cells may be available for cell-mediated immune responses against melanoma in patients that receive bevacizumab. We had too few samples to investigate whether bevacizumab truly increases the abundance of T cells reacting against melanoma-specific antigens. Recently, vemurafenib has been shown to increase the number of tumor-infiltrating lymphocytes in patients affected by unresectable Stage III or Stage IV melanoma.Citation18 One meta-analysis of multiple tumor types reported an association between CD8+ tumor-infiltrating lymphocytes and favorable overall survival, disease-specific survival and progression/disease/relapse-free survival.Citation19 Conversely, in three clinical trials involving various schedules of a gp100-derived peptide (gp100209–217)-based vaccine coupled to high-dose IL-2, there was no association between pre- and post- treatment gp100209–217- or influenza-specific CD8+ T cells.Citation20 The authors stated that these trials were not sufficiently powered to detect differences between responders and non-responders. Our data suggest that the addition of bevacizumab to chemotherapy improves peripheral CD8+ T-cell counts.
Sunitinib, a multi-tyrosine kinase inhibitor with antiangiogenic effects, has been studied in patients bearing metastatic renal cell cancer. Sunitinib was found to reduce the number of myeloid-derived suppressor cells and regulatory T cells in one group of patients.Citation21 In another study, a decrease in regulatory T cells was shown to correlate with overall survival in metastatic renal cancer patients treated with sunitinib.Citation22 Bevacizumab is also used frequently for the treatment of metastatic colon cancer. Patients treated with bevacizumab have been reported to manifest reduced levels of peripheral regulatory T cells and decreased regulatory T-cell proliferation.Citation23 Together with our findings, these observations suggest that the inhibition of angiogenesis exert immunomodulatory effects in patients affected by distinct tumor types.
We have previously shown that patients with metastatic melanoma exhibit a TH2-driven inflammatory state that is in part due to the overproduction of VEGF.Citation15 In this study, we confirmed this observation, finding that patients affected by metastatic melanoma exhibit a systemic TH2-dominant state and a TH1/TH2 cell ratio of 0.76. This ratio increased in patients who received bevacizumab together with chemotherapy while it decreased in subjects treated with chemotherapy alone, though this difference was not significant.
We found that the administration of bevacizumab together with chemotherapy significantly lowers the circulating levels of IL-6. Multiple studies have found that high circulating levels of IL-6 reduced survival and time to progression in melanoma patients.Citation24-Citation26 Moreover, the levels of IL-6 have been found to be significantly higher in the serum of patients with resected, high-risk melanoma that in that of healthy individuals.Citation27 In vitro data suggest that IL-6 stimulates the production of the TH2 cytokine IL-10 by metastatic melanoma.Citation28 This may in part explain the improvement in the TH1/TH2 cell ratio that we observed in parallel with the decrease in circulating IL-6 levels. IP-10 also decreased after two cycles of ACB- but not AC-based chemotherapy. This result is consistent with the established association between IP-10 and angiogenesis.
In summary, we observed that the addition of bevacizumab to conventional chemotherapy for the treatment of metastatic melanoma modulates cellular immunity and the secretion of pro-inflammatory cytokines. Specifically, we observed that patients who received bevacizumab had a significant increase in circulating CD8+ cells and significant decreases in serum IL-6.
Materials and Methods
Specimens
Our study was performed on specimens from patients enrolled on two clinical trials, N057e and N0775 (Table S2). There were 76 patients treated with albumin-bound paclitaxel (100 mg/m2 i.v. days 1, 8 and 15) and carboplatin (AUC 2 days 1, 8 and 15) every 28 d in the Phase II clinical trial N057e run by the North Central Cancer Treatment Group. We obtained specimens from 55 patients enrolled in N057e for our studies. Ninety-four patients were treated with temozolomide (200 mg/m2 by mouth days 1–5) and bevacizumab (10 mg/kg i.v. days 1 and 15) every 28 d, or albumin-bound paclitaxel (80–100 mg/m2 i.v. days 1, 8 and 15), carboplatin (AUC 5–6 day 1) and bevacizumab (10 mg/kg i.v. days 1 and 15) in the context of the randomized Phase II trial N0775. We obtained samples from 39 patients of these patients treated with albumin-bound paclitaxel, carboplatin and bevacizumab for our studies. Informed consent was obtained from all subjects to participate in N057e and N0775, and the Mayo Clinic Institutional Review Board approved this study.
Laboratory assays
Plasma cytokines, chemokines and growth factors were measured using the Milliplex human cytokine 42 analyte panel (Millipore Corporation) per manufacturer’s recommendations. Briefly, 25 μL of patient plasma and standards were added to antibody containing microspheres and incubated for 1 h at room temperature. Microspheres were washed and biotinylated detection antibodies added, followed by streptavidin conjugated R-phycoerythrin. Microspheres were washed and read by Luminex (Millipore). Cytokine concentrations were determined using a standard curve with a dynamic range between 1.6 and 5000 pg/mL, using Milliplex Analyst software (VigeneTech). The following anti-human monoclonal antibodies were used for cell surface staining and flow cytometry: anti-CD3-APC, FITC and PE, anti-CD4-FITC, anti-CD8-PE, anti-CD62L APC, anti-CD197 FITC, anti-CD294 Alexa Flour 647 (BD PharMingen) and anti-TIM-3 PE (R&D Systems). For surface staining, previously frozen PBMCs were thawed and 0.5 × 106−1.0 × 106 cells were aliquoted into 96 well plates in 100 μL. The desired antibody or antibody pool was added at 5 µL/well. The cells and antibodies were incubated for 30 min at 4°C and washed twice with PBS containing 0.1% bovine serum albumin (BSA) and 0.05% sodium azide (Sigma). Four-color flow cytometry was performed on a LSRII (Becton Dickenson) or Guava 8HT (Millipore Corporation) flow cytometer and the Cell Quest software (Becton Dickenson), or Guavasoft software (Millipore Corporation) was used for acquisition and analysis.
We excluded cell subsets, cytokines and growth factors with coefficients of variation greater than 30%, or if more than 30% of the samples had values above or below the limits of detection of our assays. This applied to FLT3 ligand, IL-1a, IL-3, IL-4, IL-5, IL-7, IL-9, IL-10, IL-12p40, IL-13, IL-15, platelet-derived growth factor (PDGF)-AA/AB, CCL5, sCD40L, sIL2Ra, TNFβ.
Statistical analyses
Patient samples were measured at baseline and just prior to the second (RPC1) and third cycles (RPC2) of therapy. The measurements made just prior to the third cycle were part of a restaging visit that included imaging. The relative percent change (RPC) in PBMC subsets and cytokine levels were determined for each patient. The Mann–Whitney U test was then used to compare patients who received chemotherapy without bevacizumab (AC; N057e) to those who received chemotherapy with bevacizumab (ACB; N0775). A Cox proportional hazards model was used to determine if there was a relationship between progression-free survival and RPC2. All statistical tests were two-sided. JMP 9.0.1 (SAS Institute Inc.) was used for all statistical tests. was created with GraphPad 5.01 (GraphPad Software).
Additional material
Download Zip (120.4 KB)Acknowledgments
Funding was provided by Melanoma Research Alliance.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Notes
† These authors contributed equally to this work.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62:10 - 29; http://dx.doi.org/10.3322/caac.20138; PMID: 22237781
- Mansfield AS, Markovic SN. Novel therapeutics for the treatment of metastatic melanoma. Future Oncol 2009; 5:543 - 57; http://dx.doi.org/10.2217/fon.09.15; PMID: 19450181
- Markovic SN, Erickson LA, Rao RD, Weenig RH, Pockaj BA, Bardia A, et al, Melanoma Study Group of Mayo Clinic Cancer Center. Malignant melanoma in the 21st century, part 2: staging, prognosis, and treatment. Mayo Clin Proc 2007; 82:490 - 513; http://dx.doi.org/10.4065/82.4.490; PMID: 17418079
- Markovic SN, Erickson LA, Rao RD, Weenig RH, Pockaj BA, Bardia A, et al, Melanoma Study Group of the Mayo Clinic Cancer Center. Malignant melanoma in the 21st century, part 1: epidemiology, risk factors, screening, prevention, and diagnosis. Mayo Clin Proc 2007; 82:364 - 80; PMID: 17352373
- Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711 - 23; http://dx.doi.org/10.1056/NEJMoa1003466; PMID: 20525992
- Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al, BRIM-3 Study Group. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011; 364:2507 - 16; http://dx.doi.org/10.1056/NEJMoa1103782; PMID: 21639808
- Eggermont AM, Suciu S, Santinami M, Testori A, Kruit WH, Marsden J, et al, EORTC Melanoma Group. Adjuvant therapy with pegylated interferon alfa-2b versus observation alone in resected stage III melanoma: final results of EORTC 18991, a randomised phase III trial. Lancet 2008; 372:117 - 26; http://dx.doi.org/10.1016/S0140-6736(08)61033-8; PMID: 18620949
- Rao RD, Holtan SG, Ingle JN, Croghan GA, Kottschade LA, Creagan ET, et al. Combination of paclitaxel and carboplatin as second-line therapy for patients with metastatic melanoma. Cancer 2006; 106:375 - 82; http://dx.doi.org/10.1002/cncr.21611; PMID: 16342250
- Kottschade LA, Suman VJ, Amatruda T 3rd, McWilliams RR, Mattar BI, Nikcevich DA, et al. A phase II trial of nab-paclitaxel (ABI-007) and carboplatin in patients with unresectable stage IV melanoma : a North Central Cancer Treatment Group Study, N057E(1). Cancer 2011; 117:1704 - 10; http://dx.doi.org/10.1002/cncr.25659; PMID: 21472717
- Kottschade LA, Suman VJ, Perez DG, McWilliams RR, Kaur JS, Amatruda TT 3rd, et al. A randomized phase 2 study of temozolomide and bevacizumab or nab-paclitaxel, carboplatin, and bevacizumab in patients with unresectable stage IV melanoma : a North Central Cancer Treatment Group study, N0775. Cancer 2013; 119:586 - 92; http://dx.doi.org/10.1002/cncr.27760; PMID: 22915053
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004; 350:2335 - 42; http://dx.doi.org/10.1056/NEJMoa032691; PMID: 15175435
- Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006; 355:2542 - 50; http://dx.doi.org/10.1056/NEJMoa061884; PMID: 17167137
- Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med 2003; 349:427 - 34; http://dx.doi.org/10.1056/NEJMoa021491; PMID: 12890841
- Kim KB, Sosman JA, Fruehauf JP, Linette GP, Markovic SN, McDermott DF, et al. BEAM: a randomized phase II study evaluating the activity of bevacizumab in combination with carboplatin plus paclitaxel in patients with previously untreated advanced melanoma. J Clin Oncol 2012; 30:34 - 41; http://dx.doi.org/10.1200/JCO.2011.34.6270; PMID: 22124101
- Nevala WK, Vachon CM, Leontovich AA, Scott CG, Thompson MA, Markovic SN, Melanoma Study Group of the Mayo Clinic Cancer Center. Evidence of systemic Th2-driven chronic inflammation in patients with metastatic melanoma. Clin Cancer Res 2009; 15:1931 - 9; http://dx.doi.org/10.1158/1078-0432.CCR-08-1980; PMID: 19240164
- Gabrilovich D, Ishida T, Oyama T, Ran S, Kravtsov V, Nadaf S, et al. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood 1998; 92:4150 - 66; PMID: 9834220
- Mimura K, Kono K, Takahashi A, Kawaguchi Y, Fujii H. Vascular endothelial growth factor inhibits the function of human mature dendritic cells mediated by VEGF receptor-2. Cancer Immunol Immunother 2007; 56:761 - 70; http://dx.doi.org/10.1007/s00262-006-0234-7; PMID: 17086423
- Wilmott JS, Long GV, Howle JR, Haydu LE, Sharma RN, Thompson JF, et al. Selective BRAF inhibitors induce marked T-cell infiltration into human metastatic melanoma. Clin Cancer Res 2012; 18:1386 - 94; http://dx.doi.org/10.1158/1078-0432.CCR-11-2479; PMID: 22156613
- Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer 2011; 105:93 - 103; http://dx.doi.org/10.1038/bjc.2011.189; PMID: 21629244
- Sosman JA, Carrillo C, Urba WJ, Flaherty L, Atkins MB, Clark JI, et al. Three phase II cytokine working group trials of gp100 (210M) peptide plus high-dose interleukin-2 in patients with HLA-A2-positive advanced melanoma. J Clin Oncol 2008; 26:2292 - 8; http://dx.doi.org/10.1200/JCO.2007.13.3165; PMID: 18467720
- Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res 2009; 15:2148 - 57; http://dx.doi.org/10.1158/1078-0432.CCR-08-1332; PMID: 19276286
- Adotevi O, Pere H, Ravel P, Haicheur N, Badoual C, Merillon N, et al. A decrease of regulatory T cells correlates with overall survival after sunitinib-based antiangiogenic therapy in metastatic renal cancer patients. J Immunother 2010; 33:991 - 8; http://dx.doi.org/10.1097/CJI.0b013e3181f4c208; PMID: 20948437
- Terme M, Pernot S, Marcheteau E, Sandoval F, Benhamouda N, Colussi O, et al. VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Cancer Res 2013; 73:539 - 49; http://dx.doi.org/10.1158/0008-5472.CAN-12-2325; PMID: 23108136
- Soubrane C, Rixe O, Meric JB, Khayat D, Mouawad R. Pretreatment serum interleukin-6 concentration as a prognostic factor of overall survival in metastatic malignant melanoma patients treated with biochemotherapy: a retrospective study. Melanoma Res 2005; 15:199 - 204; http://dx.doi.org/10.1097/00008390-200506000-00009; PMID: 15917702
- Tartour E, Blay JY, Dorval T, Escudier B, Mosseri V, Douillard JY, et al. Predictors of clinical response to interleukin-2--based immunotherapy in melanoma patients: a French multiinstitutional study. J Clin Oncol 1996; 14:1697 - 703; PMID: 8622090
- Mouawad R, Rixe O, Meric JB, Khayat D, Soubrane C. Serum interleukin-6 concentrations as predictive factor of time to progression in metastatic malignant melanoma patients treated by biochemotherapy: a retrospective study. Cytokines Cell Mol Ther 2002; 7:151 - 6; http://dx.doi.org/10.1080/13684730210002328; PMID: 14660055
- Yurkovetsky ZR, Kirkwood JM, Edington HD, Marrangoni AM, Velikokhatnaya L, Winans MT, et al. Multiplex analysis of serum cytokines in melanoma patients treated with interferon-alpha2b. Clin Cancer Res 2007; 13:2422 - 8; http://dx.doi.org/10.1158/1078-0432.CCR-06-1805; PMID: 17438101
- Terai M, Eto M, Young GD, Berd D, Mastrangelo MJ, Tamura Y, et al. Interleukin 6 mediates production of interleukin 10 in metastatic melanoma. Cancer Immunol Immunother 2012; 61:145 - 55; http://dx.doi.org/10.1007/s00262-011-1084-5; PMID: 21853302