Abstract
Various strategies have been developed to deliver tumor-associated antigens (TAAs) to dendritic cells (DCs). Among these, the fusion of DCs and whole cancer cells can process a broad array of TAAs, including hitherto unidentified molecules, and present them in complex with MHC Class I and II molecules and in the context of co-stimulatory signals. DC-cancer cell fusions have been shown to stimulate potent antitumor immune responses in animal models. In early clinical trials, however, the antitumor effects of DC-cancer cell fusions are not as vigorous as in preclinical settings. This mini-review summarizes recent advances in anticancer vaccines based on DC-cancer cell fusions.
Keywords: :
Dendritic cells (DCs) are potent antigen-presenting cells (APCs) that have been extensively used for the development of anticancer vaccines.Citation1 Among various strategies, vaccination with DCs pulsed with specific tumor-associated antigen (TAA)-derived peptides has been intensively investigated.Citation2,Citation3 However, a major drawback of these approaches stems from the limited number of known TAAs that are available for multiple HLA molecules. In addition, the downregulation of a specific TAA has been reported as an efficient means whereby cancer cells evade TAA-targeting immune responses. Thus, various strategies have been developed to deliver as many whole TAAs as possible to DCs by means of cancer cell-derived RNA, whole cancer-cell lysates or apoptotic malignant cells.Citation2,Citation4-Citation6 Alternatively, antitumor immune responses have been obtained by fusing DCs and whole neoplastic cells and using the resulting DC/cancer cell chimera as an anticancer vaccine. In this setting, all TAAs—including known and unidentified molecules—are delivered to DCs, processed and presented to T cells in complex with MHC Class I and II molecules and in the context of co-stimulatory signals.Citation7
The fusion of a DC and a cancer cell by chemical, physical or biological means creates a heterokaryon expressing DC-derived co-stimulatory molecules, an efficient antigen-processing and -presentation machinery as well as TAAs.Citation8 Thus, at least theoretically, this method allows DCs to be exposed to the entire repertoire of TAAs originally expressed by the malignant cell, to process them endogenously and to present TAA epitopes thought the MHC Class I and II pathways to activate both CD8+ and CD4+ T cells.Citation9 Upon exposure to polyethylene glycol (PEG), DCs and cancer cells become hybrid cells sharing a unified cytoplasm but preserving the identity of their nucleiCitation10,Citation11 (). Such a configuration allows TAAs and DC-derived MHC molecules to be co-expressed, resulting in the processing of TAAs and their presentation on the cell surface, where also DC-derived co-stimulatory molecules are expressed.
Figure 1. Antigen processing and presentation by a dendritic cell fused to a cancer cell. Dendritic cell (DC)-tumor cell fusions express MHC Class I and II molecules, co-stimulatory molecules as well as tumor-associated antigens. These cell fusions are hence able to process cancer cell-derived peptides and form MHC Class I-peptide complexes in the endoplasmic reticulum, which are transported to the cell surface and presented to CD8+ T cells. Along similar lines, fused cells can synthesize MHC Class II molecules, load them with tumor-derived peptides and present these complex to CD4+ T cells. Globally, this results in the activation of potent tumor-specific cytotoxic T lymphocyte (CTL) responses.
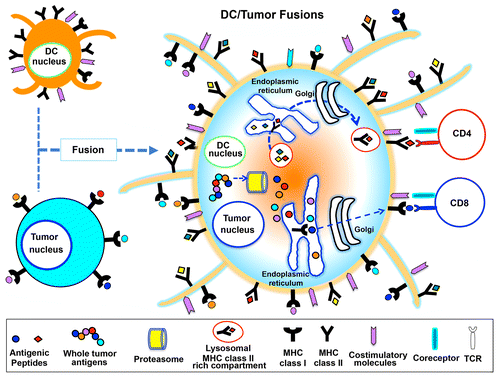
This approach offers several advantages for the presentation of TAA-derived peptides and the subsequent induction of antitumor immune responses. First, both known and unidentified TAAs are expressed (at least theoretically), circumventing the daunting task of identifying TAAs in an individualized fashion. Second, multiple TAAs can be simultaneously processed and presented on the surface of DC/cancer cell fusions, increasing the frequency of responding cytotoxic T lymphocytes (CTLs). Third, TAAs are presented in the context of abundant co-stimulatory signals, avoiding the potential induction of tolerance and maximizing antitumor immune responses. Fourth, DC/cancer cell fusions drive the simultaneous activation of polyclonal CD8+ and CD4+ tumor-specific T cells, the latter of which provide robust help for the induction and maintenance of CTLs.
A major limitation to the use of DC/cancer cell fusions is the availability of adequate amounts of autologous tumor cells, in turn stemming from the limited availability of viable tumor samples and/or technical difficulties in the culture of cancer cells. To circumvent this issue, established allogeneic cancer cell lines have been used instead of autologous tumor cells. The rationale underlying this approach is that some TAAs are shared by several tumors. We have reported that the fusion of autologous DCs with allogeneic tumor cell lines can induce antigen-specific polyclonal CTLs exerting cytotoxic activity against autologous tumor cells.Citation12 This strategy has numerous advantages. First, allogeneic cancer cell lines that are well characterized as a source of TAAs can be massively propagated in vitro under good manufacturing practice (GMP) standards. Second, this approach does not require the HLA typing of patients or allogeneic tumor cells, as DC/cancer cell fusions can process and present multiple TAAs on MHC Class I and II molecules. Therefore, the fusion of autologous DCs with allogeneic tumor cells represents a very simple approach to anticancer vaccination and—at least potentially—can be administered in a direct fashion rather than upon costly HLA typing procedures.
The clinical responses to DC/cancer cell fusion-based vaccines, however, have not been as vigorous as those recorded in animal models.Citation8,Citation13 This deceiving result reflects, at least in part, the poor immunogenicity of DCs and whole cancer cells (be them allogeneic or autologous), which generally secrete several immunosuppressive mediators including transforming growth factor β1 (TGFβ1).Citation14 A method to enhance the immunogenicity of both DCs and cancer cells is therefore required to produce immunogenic vaccines based on the DC/cancer cell fusion technology. A huge advantage of this approach is that DCs and cancer cells can be independently subjected to (genetic) manipulations for the acquisition of characters that persist after fusion. Recently, we have reported that the fusions between Toll-like receptor (TLR)-stimulated DCs and heat-treated cancer cells triggered CTL responses in vitro that were more robust than those elicited by conventional DC/cancer cell fusions.Citation13-Citation16 However, it is still unclear which specific treatments significantly improve the immunogenicity of neoplastic cells and which TLR agonists exert the greatest immunostimulatory effects in the context of DC/cancer cell fusion-based anticancer vaccines.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol 1991; 9:271 - 96; http://dx.doi.org/10.1146/annurev.iy.09.040191.001415; PMID: 1910679
- Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, et al. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med 1998; 4:328 - 32; http://dx.doi.org/10.1038/nm0398-328; PMID: 9500607
- Celluzzi CM, Mayordomo JI, Storkus WJ, Lotze MT, Falo LD Jr.. Peptide-pulsed dendritic cells induce antigen-specific CTL-mediated protective tumor immunity. J Exp Med 1996; 183:283 - 7; http://dx.doi.org/10.1084/jem.183.1.283; PMID: 8551233
- Phan V, Errington F, Cheong SC, Kottke T, Gough M, Altmann S, et al. A new genetic method to generate and isolate small, short-lived but highly potent dendritic cell-tumor cell hybrid vaccines. Nat Med 2003; 9:1215 - 9; http://dx.doi.org/10.1038/nm923; PMID: 12925849
- Paglia P, Chiodoni C, Rodolfo M, Colombo MP. Murine dendritic cells loaded in vitro with soluble protein prime cytotoxic T lymphocytes against tumor antigen in vivo. J Exp Med 1996; 183:317 - 22; http://dx.doi.org/10.1084/jem.183.1.317; PMID: 8551239
- Russo V, Tanzarella S, Dalerba P, Rigatti D, Rovere P, Villa A, et al. Dendritic cells acquire the MAGE-3 human tumor antigen from apoptotic cells and induce a class I-restricted T cell response. Proc Natl Acad Sci U S A 2000; 97:2185 - 90; http://dx.doi.org/10.1073/pnas.040540197; PMID: 10681453
- Gong J, Chen D, Kashiwaba M, Kufe D. Induction of antitumor activity by immunization with fusions of dendritic and carcinoma cells. Nat Med 1997; 3:558 - 61; http://dx.doi.org/10.1038/nm0597-558; PMID: 9142127
- Gong J, Koido S, Calderwood SK. Cell fusion: from hybridoma to dendritic cell-based vaccine. Expert Rev Vaccines 2008; 7:1055 - 68; http://dx.doi.org/10.1586/14760584.7.7.1055; PMID: 18767954
- Koido S, Tanaka Y, Chen D, Kufe D, Gong J. The kinetics of in vivo priming of CD4 and CD8 T cells by dendritic/tumor fusion cells in MUC1-transgenic mice. J Immunol 2002; 168:2111 - 7; PMID: 11859096
- Koido S, Ohana M, Liu C, Nikrui N, Durfee J, Lerner A, et al. Dendritic cells fused with human cancer cells: morphology, antigen expression, and T cell stimulation. Clin Immunol 2004; 113:261 - 9; http://dx.doi.org/10.1016/j.clim.2004.08.004; PMID: 15507391
- Koido S, Gong J. Characterization of structure and direct antigen presentation by dendritic/tumor-fused cells as cancer vaccines. Anticancer Res 2013; 33:347 - 54; PMID: 23393323
- Koido S, Hara E, Homma S, Torii A, Toyama Y, Kawahara H, et al. Dendritic cells fused with allogeneic colorectal cancer cell line present multiple colorectal cancer-specific antigens and induce antitumor immunity against autologous tumor cells. Clin Cancer Res 2005; 11:7891 - 900; http://dx.doi.org/10.1158/1078-0432.CCR-05-1330; PMID: 16278414
- Koido S, Homma S, Hara E, Namiki Y, Takahara A, Komita H, et al. Regulation of tumor immunity by tumor/dendritic cell fusions. Clin Dev Immunol 2010; 2010:516768; http://dx.doi.org/10.1155/2010/516768; PMID: 21048993
- Koido S, Homma S, Okamoto M, Namiki Y, Takakura K, Takahara A, et al. Combined TLR2/4-activated dendritic/tumor cell fusions induce augmented cytotoxic T lymphocytes. PLoS ONE 2013; 8:e59280; http://dx.doi.org/10.1371/journal.pone.0059280
- Koido S, Hara E, Homma S, Torii A, Mitsunaga M, Yanagisawa S, et al. Streptococcal preparation OK-432 promotes fusion efficiency and enhances induction of antigen-specific CTL by fusions of dendritic cells and colorectal cancer cells. J Immunol 2007; 178:613 - 22; PMID: 17182602
- Koido S, Hara E, Homma S, Mitsunaga M, Takahara A, Nagasaki E, et al. Synergistic induction of antigen-specific CTL by fusions of TLR-stimulated dendritic cells and heat-stressed tumor cells. J Immunol 2007; 179:4874 - 83; PMID: 17878387