Abstract
Regulatory T cells (Tregs) play an important role in controlling antitumor T-cell responses and hence represent a considerable obstacle for cancer immunotherapy. The abundance of specific Treg populations in cancer patients has been poorly analyzed so far. Here, we demonstrate that in breast cancer patients, Tregs often control spontaneous effector memory T-cell responses against mammaglobin, a common breast tissue-associated antigen that is overexpressed by breast carcinoma. Using functional assays, we identified a HLA-DRB1*04:01- and HLA-DRB1*07:01-restricted epitope of mammaglobin (mam34–48) that was frequently recognized by Tregs isolated from breast cancer patients. Using mam34–48-labeled HLA Class II tetramers, we quantified mammaglobin-specific Tregs and CD4+ conventional T (Tcon) cells in breast carcinoma patients as well as in healthy individuals. Both mammaglobin-specific Tregs and Tcon cells were expanded in breast cancer patients, each constituting approximately 0.2% of their respective cell subpopulations. Conversely, mammaglobin-specific Tregs and CD4+ Tcon cells were rare in healthy individuals (0.07%). Thus, we provide here for the first time evidence supporting the expansion of breast tissue-specific Tregs and CD4+ Tcon cells in breast cancer patients. In addition, we substantiate the potential implications of breast tissue-specific Tregs in the suppression of antitumor immune responses in breast cancer patients. The HLA Class II tetramers used in this study may constitute a valuable tool to elucidate the role of antigen-specific Tregs in breast cancer immunity and to monitor breast cancer-specific CD4+ T cells.
Introduction
Effector and memory T-cell responses against tumor-associated antigens (TAAs) often arise spontaneously in breast cancer patients.Citation1-Citation3 Upon appropriate reactivation, such cells can recognize autologous tumor cells and exert antitumor activity in vitro and in vivo.Citation4,Citation5 These findings suggest that preexisting tumor-specific effector T-cell responses may have an impact on natural disease progression as well as on the efficiency of clinical interventions. Indeed, the accumulation of CD4+ and CD8+ memory and effector T cells is correlated with improved disease outcomes among primary and advanced breast carcinoma patients.Citation6,Citation7 In recent years, it has become ever more clear that CD4+ T cells play an important role in antitumor immunity as they provide crucial signals for the induction of stable antitumor responses.Citation8,Citation9 However, the expansion and activity of self-reactive effector T cells is strongly limited by a population of T cells with inhibitory functions, i.e., regulatory T cells (Tregs). Tregs constitute a subset of CD4+ T cells with immunosuppressive capacity. Human Tregs are characterized by the expression of the interleukin-2 (IL-) receptor α chain (CD25), the forkhead box P3 (FOXP3) transcription factor and reduced expression levels of the interleukin-7 receptor (CD127).Citation10 Tregs often accumulate in the blood and malignant lesions of cancer patients. Robust tumor infiltration by Tregs correlates with poor survival rates in patients affected by distinct tumor types including (but not limited to) breast,Citation11 gastricCitation12 and ovarian cancer.Citation13
Since Tregs may suppress not only spontaneous antitumor T-cell responses but also T-cell responses induced by immunotherapeutic interventions, such as anticancer vaccines, this immunosuppressive cell population has a critical clinical relevance. Tregs are activated upon recognizing cognate antigens through the T-cell receptor, hence acquiring robust immunosuppressive functions.Citation14,Citation15 It is therefore conceivable that in cancer patients, expanded populations of TAA-specific Tregs might be particularly active in suppressing antitumor immune responses. Expanded populations of TAA-specific Tregs may have a strong negative impact in the setting of anticancer vaccination, since TAA-targeting vaccines might specifically reactivate preexisting tumor-reactive Tregs and hence potentially exert adverse rather than protective effects. In line with this assumption, it has previously been demonstrated, in mice, that TAAs can induce Tregs that inhibit antitumor immune responses in the periphery.Citation16
Thus, monitoring TAA-specific Tregs should constitute a crucial part of any clinical study of cancer immunotherapy, especially when clinical procedures attempt to activate CD4+ T cells, as this bears the risk of co-activating CD4+ immunosuppressive Tregs. So far, the specificities of human Tregs have been very poorly investigated, in particular among breast cancer patients. In part, this may be due to a lack of appropriate experimental tools, notable the lack of HLA Class II tetramers labeled with HLA Class II-restricted epitopes of relevant TAAs.
Here, using dedicated functional assays, we characterized HLA Class II-restricted epitopes of the breast cancer-associated antigen mammaglobin and generated HLA Class II tetramers to analyze the presence, frequency and biological relevance of mammaglobin-specific Tregs in breast cancer patients. Mammaglobin is highly expressed by all breast tumors, and its expression is largely confined to the breast tissue,Citation17 rendering it a useful diagnostic marker as well as a prominent therapeutic target.Citation18-Citation20 Previous studies from our group have revealed that mammaglobin is an important target of spontaneous memory and effector T-cell responses in breast cancer patients. Although mammaglobin is well studied, no specific HLA Class II tetramer exists as of yet. In the present study, tetramers of two different HLA Class II molecules presenting a mammaglobin-derived peptide were generated, tested and employed to stain T cells isolated from breast cancer patients.
Results
Presence of mammaglobin-specific effector and regulatory T cells in breast cancer patients
In a first set of experiments, we determined the presence of mammaglobin-reactive T cells in the blood of 23 breast cancer patients ex vivo by interferon γ (IFNγ) ELISPOT assays. To this end, we co-cultured patient-derived T cells together with autologous dendritic cells (DCs) that were pulsed with 200 μg/mL of a synthetic long peptide derived from the mammaglobin epitope 4–56 () or with human IgG, as negative control antigen, at 5:1 ratio for 40 h in ELISPOT plates. In order to assess the influence of Tregs on mammaglobin-specific T-cell reactivity, cell aliquots from 16 samples were depleted of Tregs via anti-CD25 magnetic bead separation. Original results from two representative patients are shown in . These demonstrate the presence of mammaglobin-reactive T cells in both Treg- depleted samples, while mammaglobin-specific effector T-cell reactivity was completely () or partially () inhibited in the presence of Tregs. Overall, we detected significant effector T-cell responsiveness in 50% of the samples that were depleted of Tregs, but only in 35% of the samples that contained them (). Moreover, we observed a significant increase in the number of mammaglobin-reactive effector T cells upon Treg depletion (138 IFNγ spots/well vs. 76 IFNγ spots/well in total T-cell population; p = 0.02). These data demonstrate that pre-existing mammaglobin-reactive effector T cells from breast carcinoma patients are functionally controlled by Tregs upon mammaglobin-specific reactivation.
Figure 1. Functional assays of mammaglobin-specific conventional and regulatory T cells. (A) Position of tested peptides within the mammaglobin sequence. (B andC) Total T cells or T cells depleted of regualtory T cells (Tregs) were analyzed in an interferon γ (IFNγ) ELISPOT assay upon stimulation with the mammaglobin-derived peptide mam4–56 or human IgG (negative control). The assay was conducted in triplicate instances. Representative results from two patients are shown (p values as per two-tailed Student's t-tests are indicated). (D) Frequency of patients demonstrating a significant mammaglobin-specific activation of T cells in ELISPOT assays. Response rate of total T cells (23 patients tested) and Treg-depleted T cells (16 patients tested) are shown. (E and F) Analysis of mammaglobin-specific circulating Tregs from breast carcinoma patients. Treg cells were stimulated with dendritic cells pulsed with the peptides of interest (or with IgG40–89, as a negeative control) and polyclonally activated conventional T (Tcon) cells were added after 18 h. The proliferation of Tcon cells was measured by thymidine uptake assays. As a control, the proliferation of Tcon cells in the absence of Tregs is shown. Assays were conducted in triplicate instances. Results are reported as means of triplicate assays ± SEM (p values as per two-tailed Student's t-tests are indicated). In (E), representative results from one patient exhibiting Tregs specific for mam34–48, mam42–57 and mam4–56 are shown. (F) illustrates the percentage of patients exhibiting a significant activation of Tregs upon stimulation with the indicated peptide (n = number of patients tested). (G) Peptide-specific Treg-mediated suppression of Tcon proliferation, calculated as a percent reduction in the proliferation of polyclonally activated Tcon cells incubated with Tregs stimulated with mammaglobin-derived vs. control peptides. Red symbols represent a significant activation in Treg specificity assays (p values as per Wilcoxon signed-rank tests comparing the median to zero are indicated).
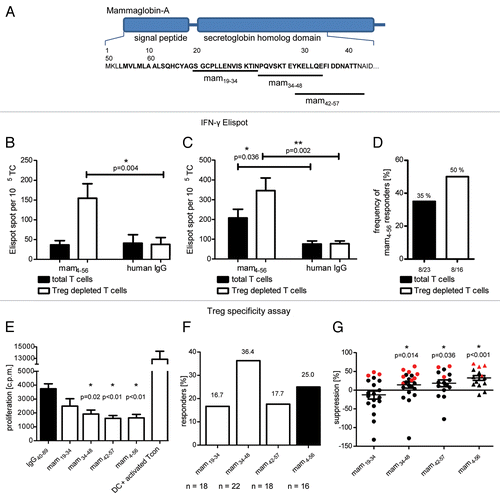
We therefore assessed the presence of mammaglobin-reactive Tregs in the blood of breast cancer patients. To this end, we stimulated purified Tregs with mammaglobin-derived synthetic peptides or control antigens (IgG) and assessed their capacity to suppress the proliferation of polyclonally activated, autologous conventional T (Tcon) cells.Citation21 For Treg activation, we either used the long synthetic peptide mam4–56 or potential HLA-DRB1-restricted epitopes thereof, which we predicted by means of the bioinformatic tool SYFPEITHY.Citation22 The prediction of 15-mers was also made for HLA-DRB1 molecules common among the Caucasian population, namely, 01:01, 03:01, 04:01, 07:01 and 15:01 (). Three mammaglobin 15-mers with the highest SYFPEITHY score had already been reported to constitute epitopes for Tcon cellsCitation23 and were therefore selected for the activation of Tregs in Treg specificity assays. All these peptides are located at the N-terminus of the secretoglobin homology domain of mammaglobin ().
Table 1. Prediction of possible HLA ligands of the known tumor-associated antigen mammaglobin*
We detected mammaglobin-reactive Tregs in up to 36% of samples tested. A representative result from a patient showing a significant activation of Tregs (as indicated by the inhibition of Tcon cell proliferation) by the predicted peptides mam34–48 and mam42–57 as well as by the long peptide mam4–56 (virtually containing all other sequences tested) is shown in . Of predicted epitopes, mam37–48 induced significant immunosuppression in the highest portion of patients tested, i.e., 36.4%. Mam19–34 and mam42–57 activated Treg immunosuppression in 16.7% and 17.7% of patients tested, respectively. The long peptide mam4–56 exert immunosuppressive effects in 25.0% of patients tested (). The extent of mammaglobin-specific Tcon suppression in patients was calculated as the percentage reduction of Tcon proliferation as mediated by Tregs activated with the peptide of interest as compared with that induced by Tregs exposed to a human IgG-derived (control) peptide.
Patients exhibited large interindividual differences in the immunosuppressive activity of mammaglobin-specific Tregs, the highest activity being a 63–71% reduction in Tcon proliferation by Tregs exposed to mam37–48, mam42–57 and mam4–56 (). On average, Tregs reactivated with these peptides inhibited Tcon proliferation by 14%, 19% and 33%, respectively. On this basis, we selected mam34–48 for generation of HLA Class II tetramers, as it significantly activated Tregs in the highest percentage of patients (36.4%) and showed a significant Tcon-suppressive activity among all patients tested.
In order to identify suitable HLA-DRB1 alleles for the generation of a mam34–48 presenting tetramer, we first compared the HLA-DRB1 alleles expressed by all patients tested in Treg specificity assays with the frequency of patients exhibiting significant peptide-specific Treg responses (). Mam34–48 showed a bias toward the predicted HLA alleles as peptide-specific Treg-mediated immunosuppression was detected in 36.4% of patients carrying either of these alleles, while Treg responsiveness was significantly reduced in patients carrying other HLA-DRB1 alleles (). We selected the HLA-DRB1* alleles 04:01 and 07:01 for the generation of mam34–48 tetrameric complexes, since (1) the binding of mam34–48 to these alleles has previously been demonstrated,Citation23 and (2) we detected mam34–48-specific Tregs in a high percentage of patients carrying either of these alleles.
Table 2. HLA restriction of mam34–48-specific Treg activation*
Enrichment of mammaglobin-specific CD4+ Tcon cells and Tregs in breast cancer patients
Both mam34–48-specific HLA-DRB1*04:01 and *07:01 tetramers were produced and kindly provided by the NIH Tetramer Core Facility. To establish optimal tetramer staining conditions, we generated mam34–48-specific CD4+ effector T-cell lines from a healthy HLA-DRB1*04:01-positive donor through repetitive antigen stimulation. After two primary stimulations in 96-well plates, the cells of each well were tested with IFNγ ELISPOT assays for their ability to recognize mam34–48. To exclude unspecific activation, the cells in each well were also tested with a negative control peptide. Cells showing the highest mam34–48-specific activation () were cloned by limiting dilution and expanded again. After two weeks, a second IFNγ ELISPOT analysis was performed to identify a T-cell line with a high mam34–48-specific IFNγ production (Cell line 1; ), which served as a positive control for tetramer staining, as well as a cell line demonstrating no antigen-specific activation (Cell line 2; ), which subsequently served as a negative control.
Figure 2. Tetramer staining of tumor-specific T cells. (A) T cells specific for the mammaglobin-derived peptide mam34–48 were induced from the CD4+ T cells of a HLA-DRB1*04:01-positive subjected by means of peptide-pulsed, autologous dendritic cells. In a first test, a cell population showing strong mam34–48-specific responses in interferon γ (IFNγ) ELISPOT assays upon mam34–48-specific activation was selected. This population was subcloned and further expanded. In a second test, a cell line showing strong mam34–48-specific IFNγ ELISPOT responses (Cell line 1) and one showing no response (Cell line 2) were selected for tetramer staining (red dots indicate selected T-cell populations). (B) Cell lines 1 (upper panels) and 2 (lower panels) were stained with a control tetramer presenting the CLIP peptide (left panels) or a tetramer presenting mam34–48 (right panels). The percentage of tetramer-positive cells is reported in each dot plot.
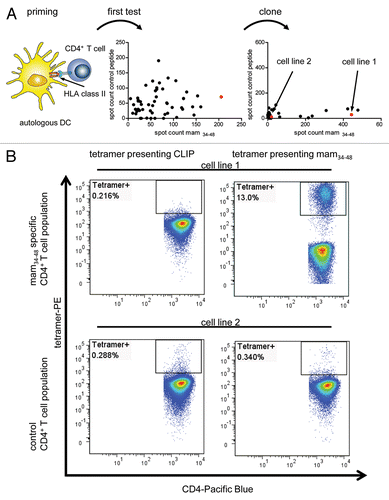
We then stained both T-cell lines with the HLA-DRB1*04:01 tetramer presenting mam34–48 as well as with a negative control tetramer presenting a Class II-associated invariant chain (CLIP)-derived peptide (). Of note, the CLIP peptide-presenting tetramer only differed from the mam34–48-presenting tetramer in the presented epitope. The mam34–48-presenting tetramer clearly labeled a population of cells within the mam34–48-specific T-cell line 1, but not within the control T-cell line 2. In addition, neither the mam34–48-specific nor the control cell line reacted with the CLIP-presenting tetramer, demonstrating the strict antigen specificity of tetramer binding.
We then assessed the presence and frequency of mammaglobin-specific CD4+ Tcon cells and Tregs in the blood of HLA-DRB1*04:01 or HLA-DRB1*07:01-positive primary breast carcinoma patients. One representative result showing a population of 0.2% tetramer-binding CD4+ T cells is shown in . Tregs and Tcon cells were further differentiated by co-staining with antibodies specific CD25, CD127 and FOXP3. Tregs were defined as CD25highCD127lowFOXP3+ cells (). Of note, tetramer-positive cells were detectable among both Tregs and Tcon cells, as shown for one representative patient in , with 5.2% of tetramer-positive cells being CD25highCD127lowFOXP3+ Tregs and 87.4% Tcon cells.
Figure 3. Tetramer staining of conventional and regulatory T cells from breast cancer patients. (A–G) Peripheral blood mononuclear cells (PBMCs) of a breast cancer patient were analyzed, upon gating on living CD3+CD4+ T cells. The patient sample was stained with tetramers presenting either mam34–48 (B) or the CLIP peptide (C). Numbers indicate the percentage of cells in the respective gate, referring to lymphocytes (A) or CD3+CD4+ T cells (B) and (C). Within CD3+CD4+ T cells, regulatory T cells (Tregs) were identified as CD25highCD127low (D) and (G) and CD25highCD127lowFOXP3+ (E), (F) and (H). (G) reports the percentage of Tregs within tetramer-positive cells in a representative patient. (I) Frequency of mam34–48- and CLIP-presenting tetramer-positive cells among CD3+CD4+ T cells of breast cancer patients and healthy donors (HD) (p values as per Mann–Whitney U tests are indicated). (J and K) Frequency of mam34–48-specific Tcon (J) cells and Tregs (K) of breast cancer patients and HDs.
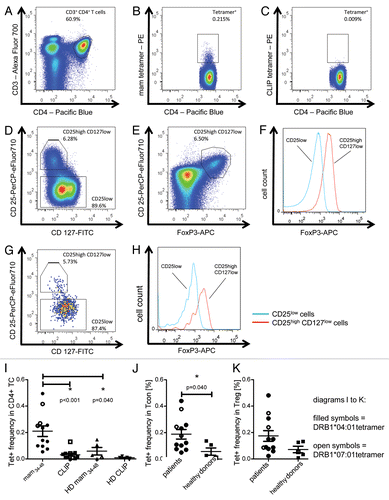
To assess the pathological significance of mammaglobin-specific CD4+ T cells in breast carcinoma, we compared the frequency of circulating mam34–48-specific CD4+ T cells in breast cancer patients and healthy subjects. To this aim, peripheral blood mononuclear cells (PBMCs) from HLA-DRB1*04:01 and *07:01-positive patients and healthy donors were stained with suitable mam34–48- and CLIP-presenting tetramers. Breast cancer patients exhibited a mean frequency of mam34–48-specific T cells of 0.21%, which was significantly higher than that of healthy donors (0.06%) (). Interestingly, mammaglobin-specific CD4+ T cells were evenly distributed among Tcon cells (0.18%) and Tregs (0.17%) in breast carcinoma patients. Similar observations held true for healthy donors (0.06% and 0.07% of mammaglobin-specific CD4+ T cells among Tcon cells and Tregs, respectively).
Discussion
We undertook the present study to analyze whether breast tissue-specific Tregs accumulate in breast carcinoma patients and whether they may contribute to the suppression of antitumor T-cell responses. As a model of tissue-specific and breast carcinoma-associated antigen we used a mammaglobin-derived long peptide, since the expression of this protein is mainly restricted to epithelial cells of the mammary gland and strongly upregulated by breast cancers.Citation17
Half of patients tested exhibited spontaneous mammaglobin-reactive effector T cells. This demonstrates that mammaglobin represents an important target for spontaneous antitumor T-cell responses in breast cancer patients. IFNγ secretion by mammaglobin-reactive effector T cells was significantly inhibited in the presence of Tregs. Treg depletion not only increased the proportion of samples with detectable mammaglobin-reactive effector T cells from 35% to 50%, but also significantly increased the average number of mammaglobin-reactive T cells per sample (data not shown). Since this increase was only detectable in samples containing T cells stimulated with mammaglobin-pulsed DCs (but not in samples co-cultured with IgG-pulsed DCs), we hypothezised that the presentation of mammaglobin-derived peptides by DCs may have activated pre-existing mammaglobin-specific Tregs. Using a functional assay based on increased immunosuppressive potential of mammaglobin-activated Tregs, we indeed demonstrated the presence of mammaglobin-reactive Tregs in the blood of 33% of breast cancer patients tested. Using the same assay, we were able to identify a mammaglobin-derived 15-mer, mam34–48, which was previously described as a HLA Class II-restricted epitope of CD4+ Tcon cellsCitation23 and which in our study efficiently activated preexisting Tregs in 36% of patients tested.
Bioinformatic prediction algorithms, previous studiesCitation23 and frequent Treg responses suggested that mam34–48 would be presented by HLA-DRB1*0401 and *0701, pushing us to generate mammaglobin-presenting HLA-DR tetramers. We could confirm the presentation of mam34–48 by HLA-DRB1*0401 through the generation of a CD4+ T-cell line that specifically reacted with a mam34–48-presenting HLA-DRB1*0401 tetramer. Using this tetramer and one consisting of HLA-DR*0701, we could confirm not only the presence of mammaglobin-specific Tregs but also that of mammaglobin-specific CD4+ Tcon cells in the circulation of breast cancer patients. Both antigen-specific Tregs and Tcon cells were strongly (4-fold) expanded in breast cancer patients when compared with healthy individuals. Interestingly, the relative abundance of mammaglobin-specific Tregs and Tcon cells among the respective T-cell subpopulations was nearly identical (approximately 0.2%). However, Tregs only represented approximately 5–10% of total mammaglobin-specific CD4+ T cells, while Tcon 85–90%. This ratio was nearly identical to that between total Tregs and Tcon cells, both in patients and in healthy individuals. Thus, although mammaglobin-specific CD4+ T cells were overrepresented in the T-cell repertoire of breast cancer patients, there was no skewing of T cells toward either a Tcon or Treg response. Still, we demonstrated that the small population of mammaglobin-specific Treg cells possesses the potential to efficiently suppress the proliferation of polyclonally activated autologous Tcon cells. This population may also account for the efficient inhibition of IFNγ secretion by mammaglobin-reactive memory T cells that we observed in ELISPOT assays. Thus, the physiological ratio between Tregs and Tcon found in healthy individualsCitation24 appears to persist even upon the expansion of Treg responses that frequently characterizes breast cancer patients.
In contrast to other publications,Citation25,Citation26 we did not perform the peptide-specific expansion of T cells before staining. Due to this reason, the frequency of tetramer-binding cells is much lower than in the aforementioned settings. The frequencies that we detected are about ten times higher than those observed ex vivo after the NY-ESO-1-targeting vaccination reported by Ayyoub et al.Citation25 The frequencies reported in the present study are within the range expected for boosted responses (1:1,000–1:20,000) and more than 400 times higher than those expected for naïve T cells (1:200,000–1:1,000,000).Citation27 In contrast to another study by Ayyoub and collaborators,Citation26 we also detected tetramer-specific Tregs. Since in that study the overall frequencies of tetramer-binding CD4+ T cells among non-vaccinated patients was below 0.01%, antigen-specific Tregs may have remained undetected. Alternatively, Tregs specific for the HLA:peptide complex tested by Ayyoub and collaborators might not exist, although NY-ESO-1-specific Tregs have previously been reported.Citation28
Until now, TAA-specific Tregs were only detected with tetramers in melanoma patients.Citation29 Thus, ours is the first study in which tumor-specific Tregs from breast carcinoma patients have been detected by HLA Class II tetramers presenting a TAA. While functional assays have been previously used to determine the presence of TAA-reactive CD4+ effector T cells and (more recently) Tregs,Citation3,Citation21 an accurate quantification of their frequency and phenotype requires appropriate HLA Class II tetramers. Unfortunately, the availability of these reagents is still very limited, mainly due to technical difficulties. Nevertheless, improvements such as the addition of a leucine zipper to stabilize the interaction between the α and β chains have rendered the production of HLA Class II tetramers technically feasible and much more effective.Citation30 Nowadays, the greatest challenge for the application of HLA Class II tetramers to the monitoring of CD4+ T-cell responses in cancer patients is the lack of defined HLA Class II-restricted epitopes from relevant TAAs.Citation27 We employed a functional Treg assay for the identification of immunogenic mammaglobin epitopes, allowing us to develop and validate two novel HLA Class II tetramers for the detection of CD4+ Tcon and Treg responses against a breast tissue-specific antigen. We found that this epitope is presented on different HLA molecules and we detected T cells specific for various HLA:peptide complexes in patients. A similar immunodominant epitope presented by several different HLA Class II molecules is known for the influenza A strain H1N1.Citation31
In conclusion, we have reported here the first ex vivo quantitative assessment of the abundance and functional properties of breast tissue-specific CD4+ Tcon cells and Tregs while introducing two new HLA Class II tetramers specific for an immunogenic epitope of mammaglobin. Our study provides further insights into the frequency of TAA-specific CD4+ Tcon and Tregs in cancer patients. In addition, we established reagents that allow for the monitoring of CD4+ T-cell responses in breast cancer patients before and after (immuno)therapeutic interventions as well as for a detailed phenotypic and functional analysis of mammaglobin-specific CD4+ T cells.
Materials and Methods
Patients
Peripheral blood samples were obtained from primary breast cancer patients and healthy donors after informed consent. The protocol was approved by the Ethical Committee of the University of Heidelberg. Patients were typed for the HLA-DRB1 locus by the Department of Immunology of the University of Heidelberg via PCR.
Antigens
Possible HLA ligands of the N-terminus of mammaglobin were predicted with the bioinformatic tool SYFPEITHI.Citation22 Top-scoring ligands has previously been published.Citation23 We used the following peptides in our study: mam13–34 (GSGCPLLENVISKTIN), mam34–48 (NPQVSKTEYKELLQE), mam42–57 (YKELLQEFIDDNATTN) and the long peptide mam4–56 (LMVLMLAALSQHCYAGSGCPLLENVISKTINPQVSKTEYKELLQEFIDDNATT). As a negative control for Treg specificity assays, the following peptide derived from human IgGs was used: IgG40–89 (SWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQTYICNVNHKP), which has previously been shown to constitute a suitable negative control.Citation21 To demonstrate the specificity of the mam34–48-specific T-cell line, an ERBB2-derived peptide predicted to bind to HLADR*04:01 was used as a negative control (ERBB2358–372, SANIQEFAGCKKIFG). All peptides were synthesized in house.
Cell culture
Peripheral blood mononuclear cells (PBMCs) were collected from the interphase of a Biocoll (Biochrom, L 6115) gradient centrifugation of heparinized blood. For FACS analysis, cells were cultured in X-VIVO 20 medium (Lonza, BE04-448Q) for up to 5 d. For Treg specificity assays, PBMCs were plated on Petri dishes in X-VIVO 20 medium for 30 min. Afterwards, non-adherent cells were collected by repeated washing with RPMI 1640 medium (Sigma Aldrich) and further cultured in X-VIVO 20 supplemented with 100 IU/mL interleukin (IL)-2 and 60 IU/mL IL-4. Adherent monocytes were used to generate dendritic cells (DCs) according to standard protocols by culture in X-VIVO 20 medium supplemented with 560 IU/mL granulocyte macrophage colony-stimulating factor (GM-CSF) and 1000 IU/mL IL-4 for up to 7 d.
Cell purification
DCs were purified by depleting contaminating cells with Dynabeads Pan Mouse IgGs (Invitrogen, 110.42) coated with anti-CD3, anti-CD19 and anti-CD56 antibodies, according to the manufacturer’s instructions. T cells were purified from non-adherent cells with the Dynabeads Untouched Human T-Cell kit (Invitrogen, 113.44D), as per manufacturer’s instructions. Regulatory T cells (Tregs) were isolated from purified T cells by means of the MACS CD4+CD25+ Treg Isolation Kit (Miltenyi Biotec, 130-091-301). CD8+ and CD4+CD25− T cells were pooled and used as conventional T (Tconv) cells for polyclonal activation.
IFNγ ELISPOT assays
Interferon γ (IFNγ)-secreting T cells were detected by means of IFNγ ELISPOT assays, conducted as previously described.Citation4 Thus, antigen-pulsed DCs were incubated with either total T cells or Treg-depleted T cells in a 1:5 a ratio for 40 h. IFNγ was visualized with the Human IFNγ ELISPOT kit (Mabtech, 3420-2A). According to the manufacturer’s protocol, the substrate was allowed to incubate for 20–50 min in the dark. The reaction was stopped by three washes with ddH2O, and membranes were allowed to dry for 72 h in the dark. Plates were read with a CTL ImmunoSpot reader (CTL Technologies) and analyzed with the ImmunoSpot Software (CTL Technologies). Assays were conducted in triplicate instances for each peptide. Patients were designated as responders when the number of spots detected in experimental conditions was significantly higher than that obtained in control wells, involving DCs pulsed with human IgGs (Sandoglobulin, CSL Behring, PZN-0571760).
Treg specificity assays
Treg specificity assays wese conducted as previously described,Citation21 with few modifications. DCs (5 × 103) were pulsed with peptides at a final concentration of 50 μg/mL for 2 h. Afterwards, purified CD4+CD25+ Tregs were added to a Treg:DC ratio = 5:1. In parallel, Tconv cells were polyclonally activated by plate-bound anti-CD3 antibodiers for 18 h. Activated Tconv cells were added to Tregs and pulsed DCs to a 1:1 Tconv:Treg ratio. All cells were cultured in X-VIVO 20 medium without supplements. After 72 h, 1 μCi 3H thymidine was added and cells were incubated for additional 18 h. Thereafter, cells were lysed by two freeze-thaw cycles and the amount of thymidine taken up by the cells was measured with a scintillation counter. Tregs were designated to be specifically activated (by DC-presented peptides) when the counts in experimental conditions were significantly (p ≤ 0.05, two-tailed Student’s t-test) reduced as compared with negative controls, involving DCs pulsed with a human IgG-derived peptide. All assays were conducted in triplicate instances. The extent of T con suppression by peptide specific Treg was calculated as a percent reduction in the proliferation of polyclonally activated Tcon cells incubated with Tregs stimulated with mammaglobin or control peptides. The average suppression after stimulation with the different peptides was tested for significance by the Wilcoxon signed-rank test comparing the median to zero.
Mammaglobin-specific T-cell lines
Monocyte-derived DCs from an HLA-DRB1*04:01-expressing healthy individuals were pulsed with 5 ng/mL mam34–48 and used to stimulate purified autologous CD4+ T cells in 96-well plates. After one week, T cells were re-stimulated with autologous PBMCs pulsed with 5 ng/mL mam34–48. In a first IFNγ ELISPOT assay, T-cell populations specifically activated by mam34–48 were identified. To this aim, DCs were pulsed with 2 μg/mL mam34–48 or an irrelevant ERBB2-derived peptide. A cell population showing robust mam34–48-specific responses but little response upon stimulation with the irrelevant peptide was diluted and plated with an average of 10 cells per well. After two weeks, cells were tested in a second IFNγ ELISPOT assay, and a T-cell line demonstrating a strong mam34–48-specific response along with one that demonstrated no specific response were used to establish optimal tetramer staining conditions.
Tetramer and FACS analysis
One million PBMCs were stained with 1 μL of the yellow LIVE/DEAD Fixable Dead Cell staining Kit solution (Invitrogen, L34959) in 100 μL FACS buffer (PBS supplemented with 2% fetal calf serum) for 20 min at 4 °C. After washing, cells were incubated with 6 μg/mL phycoerythrin (PE)-labeled tetramers loaded with either mam34–48 or a Class II-associated invariant chain (CLIP)-derived peptide in 50 μL FACS buffer for 2 h at 37 °C. Cells were washed and Fc receptors blocked with 1.5 mg/mL polyclonal human immunoglobulin (Sandoglobulin, CSL Behring, PZN-0571760). Thereafter, cells were stained with anti-CD3-Alexa Fluor 700 (Invitrogen, CD0329), anti-CD4-Pacific Blue (BD, 558116), anti-CD25-PerCP-eFluor710 (eBiosciences, 46-0257-42) and anti-CD127-FITC (BD, 560549) antibodies for 20 min at 4 °C. For intracellular FOXP3 staining, cells were permeabilized with the FOXP3 Fixation/Permeabilization Concentrate and Diluent (eBioscience, 00-5521-00) according to the manufacturer’s protocol, followed by staining with anti-FOXP3-APC antibody (eBioscience, 17-4777-42). Cells were analyzed on a FACSCanto II flow cytometer (BD Biosciences) and FlowJo software (TreeStar).
Statistical analyses
Treg specificity assays and IFNγ ELISPOT analysis were conducted in triplicate instances. T-cell responses against test peptides were compared with responses against human IgGs or a peptide thereof, which served as a negative control. Results were analyzed using two-tailed Student’s t-test. Cumulative FACS results were compared using two-tailed Mann–Whitney U test. Differences were considered statistically significant when p ≤ 0.05.
| Abbreviations: | ||
| DC | = | dendritic cell |
| FOXP3 | = | forkhead box P3 |
| IFNγ | = | interferon γ |
| IL | = | interleukin |
| GM-CSF | = | granulocyte macrophage colony-stimulating factor |
| TAA | = | tumor-associated antigen |
| Tcon | = | conventional T |
| Treg | = | regulatory T cell |
Acknowledgments
This work was supported by grants from Deutsche Forschungsgemeinschaft (SFB 938). We would like to thank the NIH Tetramer Core Facility for providing the HLA Class II tetramers.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Domschke C, Schuetz F, Ge Y, Seibel T, Falk C, Brors B, et al. Intratumoral cytokines and tumor cell biology determine spontaneous breast cancer-specific immune responses and their correlation to prognosis. Cancer Res 2009; 69:8420 - 8; http://dx.doi.org/10.1158/0008-5472.CAN-09-1627; PMID: 19843863
- Domschke C, Schuetz F, Sommerfeldt N, Rom J, Scharf A, Sohn C, et al. Effects of distant metastasis and peripheral CA 15-3 on the induction of spontaneous T cell responses in breast cancer patients. Cancer Immunol Immunother 2010; 59:479 - 86; http://dx.doi.org/10.1007/s00262-009-0801-9; PMID: 19957084
- Sommerfeldt N, Schütz F, Sohn C, Förster J, Schirrmacher V, Beckhove P. The shaping of a polyvalent and highly individual T-cell repertoire in the bone marrow of breast cancer patients. Cancer Res 2006; 66:8258 - 65; http://dx.doi.org/10.1158/0008-5472.CAN-05-4201; PMID: 16912206
- Feuerer M, Beckhove P, Bai L, Solomayer EF, Bastert G, Diel IJ, et al. Therapy of human tumors in NOD/SCID mice with patient-derived reactivated memory T cells from bone marrow. Nat Med 2001; 7:452 - 8; http://dx.doi.org/10.1038/86523; PMID: 11283672
- Schuetz F, Ehlert K, Ge Y, Schneeweiss A, Rom J, Inzkirweli N, et al. Treatment of advanced metastasized breast cancer with bone marrow-derived tumour-reactive memory T cells: a pilot clinical study. Cancer Immunol Immunother 2009; 58:887 - 900; http://dx.doi.org/10.1007/s00262-008-0605-3; PMID: 18998129
- Kim ST, Jeong H, Woo OH, Seo JH, Kim A, Lee ES, et al. Tumor-infiltrating Lymphocytes, Tumor Characteristics, and Recurrence in Patients With Early Breast Cancer. Am J Clin Oncol 2012; In press http://dx.doi.org/10.1097/COC.0b013e3182467d90; PMID: 22495453
- Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol 2011; 29:1949 - 55; http://dx.doi.org/10.1200/JCO.2010.30.5037; PMID: 21483002
- Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H. The central role of CD4(+) T cells in the antitumor immune response. J Exp Med 1998; 188:2357 - 68; http://dx.doi.org/10.1084/jem.188.12.2357; PMID: 9858522
- Knutson KL, Disis ML. Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunol Immunother 2005; 54:721 - 8; http://dx.doi.org/10.1007/s00262-004-0653-2; PMID: 16010587
- Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med 2006; 203:1693 - 700; http://dx.doi.org/10.1084/jem.20060468; PMID: 16818676
- Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, Harris AL, et al. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol 2006; 24:5373 - 80; http://dx.doi.org/10.1200/JCO.2006.05.9584; PMID: 17135638
- Sasada T, Kimura M, Yoshida Y, Kanai M, Takabayashi A. CD4+CD25+ regulatory T cells in patients with gastrointestinal malignancies: possible involvement of regulatory T cells in disease progression. Cancer 2003; 98:1089 - 99; http://dx.doi.org/10.1002/cncr.11618; PMID: 12942579
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 2004; 10:942 - 9; http://dx.doi.org/10.1038/nm1093; PMID: 15322536
- Samy ET, Parker LA, Sharp CP, Tung KS. Continuous control of autoimmune disease by antigen-dependent polyclonal CD4+CD25+ regulatory T cells in the regional lymph node. J Exp Med 2005; 202:771 - 81; http://dx.doi.org/10.1084/jem.20041033; PMID: 16172257
- Tarbell KV, Yamazaki S, Olson K, Toy P, Steinman RM. CD25+ CD4+ T cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes. J Exp Med 2004; 199:1467 - 77; http://dx.doi.org/10.1084/jem.20040180; PMID: 15184500
- Schreiber TH, Wolf D, Bodero M, Podack E. Tumor antigen specific iTreg accumulate in the tumor microenvironment and suppress therapeutic vaccination. Oncoimmunology 2012; 1:642 - 8; http://dx.doi.org/10.4161/onci.20298; PMID: 22934256
- Watson MA, Fleming TP. Mammaglobin, a mammary-specific member of the uteroglobin gene family, is overexpressed in human breast cancer. Cancer Res 1996; 56:860 - 5; PMID: 8631025
- Goedegebuure PS, Watson MA, Viehl CT, Fleming TP. Mammaglobin-based strategies for treatment of breast cancer. Curr Cancer Drug Targets 2004; 4:531 - 42; http://dx.doi.org/10.2174/1568009043332862; PMID: 15462037
- Li G, Zhang J, Jin K, He K, Wang H, Lu H, et al. Human mammaglobin: a superior marker for reverse-transcriptase PCR in detecting circulating tumor cells in breast cancer patients. Biomark Med 2011; 5:249 - 60; http://dx.doi.org/10.2217/bmm.11.20; PMID: 21473729
- Zehentner BK, Carter D. Mammaglobin: a candidate diagnostic marker for breast cancer. Clin Biochem 2004; 37:249 - 57; http://dx.doi.org/10.1016/j.clinbiochem.2003.11.005; PMID: 15003725
- Bonertz A, Weitz J, Pietsch DH, Rahbari NN, Schlude C, Ge Y, et al. Antigen-specific Tregs control T cell responses against a limited repertoire of tumor antigens in patients with colorectal carcinoma. J Clin Invest 2009; 119:3311 - 21; PMID: 19809157
- Rammensee H, Bachmann J, Emmerich NP, Bachor OA, Stevanović S. SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics 1999; 50:213 - 9; http://dx.doi.org/10.1007/s002510050595; PMID: 10602881
- Viehl CT, Frey DM, Phommaly C, Chen T, Fleming TP, Gillanders WE, et al. Generation of mammaglobin-A-specific CD4 T cells and identification of candidate CD4 epitopes for breast cancer vaccine strategies. Breast Cancer Res Treat 2008; 109:305 - 14; http://dx.doi.org/10.1007/s10549-007-9657-x; PMID: 17653857
- Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med 2005; 201:723 - 35; http://dx.doi.org/10.1084/jem.20041982; PMID: 15753206
- Ayyoub M, Dojcinovic D, Pignon P, Raimbaud I, Schmidt J, Luescher I, et al. Monitoring of NY-ESO-1 specific CD4+ T cells using molecularly defined MHC class II/His-tag-peptide tetramers. Proc Natl Acad Sci U S A 2010; 107:7437 - 42; http://dx.doi.org/10.1073/pnas.1001322107; PMID: 20368442
- Redjimi N, Duperrier-Amouriaux K, Raimbaud I, Luescher I, Dojcinovic D, Classe JM, et al. NY-ESO-1-specific circulating CD4+ T cells in ovarian cancer patients are prevalently T(H)1 type cells undetectable in the CD25+ FOXP3+ Treg compartment. PLoS One 2011; 6:e22845; http://dx.doi.org/10.1371/journal.pone.0022845; PMID: 21829534
- Nepom GT. MHC class II tetramers. J Immunol 2012; 188:2477 - 82; http://dx.doi.org/10.4049/jimmunol.1102398; PMID: 22389204
- Ebert LM, MacRaild SE, Zanker D, Davis ID, Cebon J, Chen W. A cancer vaccine induces expansion of NY-ESO-1-specific regulatory T cells in patients with advanced melanoma. PLoS One 2012; 7:e48424; http://dx.doi.org/10.1371/journal.pone.0048424; PMID: 23110239
- Jandus C, Bioley G, Dojcinovic D, Derré L, Baitsch L, Wieckowski S, et al. Tumor antigen-specific FOXP3+ CD4 T cells identified in human metastatic melanoma: peptide vaccination results in selective expansion of Th1-like counterparts. Cancer Res 2009; 69:8085 - 93; http://dx.doi.org/10.1158/0008-5472.CAN-09-2226; PMID: 19808957
- Kalandadze A, Galleno M, Foncerrada L, Strominger JL, Wucherpfennig KW. Expression of recombinant HLA-DR2 molecules. Replacement of the hydrophobic transmembrane region by a leucine zipper dimerization motif allows the assembly and secretion of soluble DR alpha beta heterodimers. J Biol Chem 1996; 271:20156 - 62; http://dx.doi.org/10.1074/jbc.271.33.20156; PMID: 8702739
- Ye M, Kasey S, Khurana S, Nguyen NT, Schubert S, Nugent CT, et al. MHC class II tetramers containing influenza hemagglutinin and EBV EBNA1 epitopes detect reliably specific CD4(+) T cells in healthy volunteers. Hum Immunol 2004; 65:507 - 13; http://dx.doi.org/10.1016/j.humimm.2004.02.019; PMID: 15172451