Abstract
Therapeutic antibodies may mediate antineoplastic effects by altering the biological functions of their target, by directly stimulating the demise of cancer cells or by activating antibody-dependent immune effector mechanisms. We have recently provided in vivo proof-of-concept for a “function-first” target and drug discovery platform in which antibodies against a multitude of tumor-associated antigens are screened for biological effects in a target-unbiased manner.
Antibody-based therapies have become part of the standard practice in the treatment of several types of cancer. Still, several malignancies remain incurable owing to the lack of effective drugs or to high incidence of acquired chemoresistance. The development of novel antineoplastic antibodies is therefore highly warranted. The critical question is how can we best succeed in this task.
In a recent issue of Cancer Cell, our group provided in vivo proof-of-principle for a “function-first” antibody drug discovery platform (see later), which was applied to identify an antibody targeting the intercellular adhesion molecule 1 (ICAM-1, also known as CD54) as a promising candidate for the treatment of multiple myeloma.Citation1 This finding was highly unexpected, for two reasons. First, the currently available knowledge on ICAM-1 biology did not suggest that an ICAM-1-targeting antibody would directly trigger the death of cancer cells. Second, it was not predictable that engaging tumor-associated macrophages by an ICAM-1-targeting antibody would mediate significant antineoplastic activity as compared with the therapeutic strategies that were currently used in advanced experimental models of multiple myeloma. In other words, as discussed in detail below, our ICAM-1-specific antibody (named BI-505) would not have been identified through traditional approaches focused on altering the biological functions of antibody targets.
The vast majority of currently approved drugs, including antibodies, has been raised against targets pre-selected for their tumorigenic activity (e.g., mitogenic signal transducers, promoters of metastatic spread, chemoresistance and resistance to stress), based on the assumption that these agents would principally act by altering the biological functions of their targets by blocking ligand-receptor interactions and downstream signaling pathways. We refer to these functions as "target biology effects." While this approach has generated therapeutic antibodies against various targets (e.g., ERBB2/HER2, EGFR, CTLA-4), accumulating preclinical and clinical evidence suggests that a significant fraction of the antineoplastic activity of these antibodies may stem from their ability to activate/modulate innate and adaptive immune responses.Citation2-Citation5 Other antibodies, such as the CD20-targeting molecule obinutuzumab (in development by Genentech/Roche), have been selected based on their ability to deliver lethal signals that were not known to ensue interaction of the receptor with native ligands.Citation6 Both these biological effects do not rely on interferences with signal transduction cascades mediated by the antibody target. Rather, these "antibody biology effects" result from active signaling pathways elicited either by the interaction of antibodies with Fcγ receptor (FcγR)-expressing immune cells or by the oligomerization (cross-linking) of receptors as promoted by the divalent format of standard antibodies. Thus, it appears unlikely that similar effects can be achieved with chemical inhibitors of receptor-conveyed signals. Importantly, antibody biology effects are highly dynamic and cannot easily be predicted from the biology of their targets. Thus, antibodies against the same receptor may operate via different mechanisms-of-action, the nature of which may determine their efficacy in an affinity-independent and isotype-independent manner.Citation7
What are the implications of these observations for contemporary antibody developers who are in possession of ever larger and more diversified libraries? Assuming that antibody libraries contain a handful of antibodies that are more efficient (against a given type of cancer) and better tolerated than others, the observations above suggest that—to maximize the chances of identifying these golden nuggets—one would want to functionally screen all the antibodies of the library that are specific for cancer-specific targets, the most interesting of which may be unknown. This poses a particular and not insignificant challenge. We have embarked in this daunting journey by developing a “function-first” approach. Such a platform relies on a differential cell-based screening methodology, which in a first step allows for the isolation of antibodies based on the targeting of a specific cell type over others, for instance a malignant cell over its normal counterpart.Citation8 Antibodies against tumor-associated targets are then screened in clinically and mechanistically relevant, high-throughput, functional in vitro assays. Finally, antibody targets are deconvoluted and the most biologically and therapeutically promising antibodies are tested in state-of-the-art in vivo experimental models.
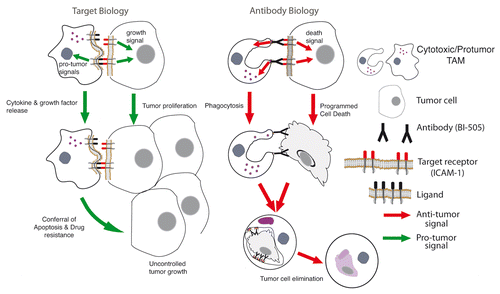
In our view, in order for the next generation of antibodies to translate into significant clinical benefits, these agents will have to both trigger profound antibody biology functions and interfere with target biology and critical disease-relevant signaling pathways (). This appears to be the case for BI-505. The antibody mediates robust biological antibody biology effects including the activation of cancer cell death and macrophage-mediated FcγR-dependent anti-myeloma immunity. Macrophages are abundant in the myeloma microenvironment, where they normally promote disease progression by delivering important survival signals to myeloma cells.Citation9 Recent findings implicate the expression of ICAM- by myeloma cells in the development of macrophage-mediated chemoresistance,Citation10 the currently unavoidable end-stage of this disease. Thus, while these are early days for BI-505 and little is known about its clinical activity, the possibility that BI-505 might reprogram tumor-associated macrophages by interacting with ICAM-1 on myeloma cells, de facto exerting antibody biology functions and intervening with target biology disease-relevant signaling pathways, offers a new therapeutic avenue against multiple myeloma.
Figure 1. Antibodies may exert antineoplastic activity by altering the biological functions of their targets and/or by mediating (direct or immune system-dependent) tumoricidal effects. In the absence of specific antibodies (e.g., BI-505), receptors (e.g., intercellular adhesion molecule 1, ICAM1) can be normally activated and transduce tumorigenic signals, hence promoting the proliferation of malignant cells and favoring their resistance to chemotherapy-induced cell death. Conversely, malignant cells coated by BI-505 not only cannot receive ICAM-1-transduced signals and undergo cell death, but also attract Fcγ receptor (FcγR)-expressing macrophages that mediate the phagocytic clearance of their corpses.
Disclosure of Potential Conflicts of Interest
B.F. is a full-time employee of BioInvent International.
References
- Veitonmäki N, Hansson M, Zhan F, Sundberg A, Löfstedt T, Ljungars A, et al. A human icam-1 antibody isolated by a function-first approach has potent macrophage-dependent antimyeloma activity in vivo. Cancer Cell 2013; 23:502 - 15; http://dx.doi.org/10.1016/j.ccr.2013.02.026; PMID: 23597564
- Selby MJ, Engelhardt JJ, Quigley M, Henning KA, Chen TH, Srinivasan M, et al. Anti-CTLA-4 Antibodies of IgG2a Isotype Enhance Antitumor Activity through Reduction of Intratumoral Regulatory T Cells. Cancer Immunol Res 2013; http://dx.doi.org/10.1158/2326-6066.CIR-13-0013
- Park S, Jiang Z, Mortenson ED, Deng L, Radkevich-Brown O, Yang X, et al. The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer Cell 2010; 18:160 - 70; http://dx.doi.org/10.1016/j.ccr.2010.06.014; PMID: 20708157
- Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med 2000; 6:443 - 6; http://dx.doi.org/10.1038/74704; PMID: 10742152
- Musolino A, Naldi N, Bortesi B, Pezzuolo D, Capelletti M, Missale G, et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol 2008; 26:1789 - 96; http://dx.doi.org/10.1200/JCO.2007.14.8957; PMID: 18347005
- Alduaij W, Ivanov A, Honeychurch J, Cheadle EJ, Potluri S, Lim SH, et al. Novel type II anti-CD20 monoclonal antibody (GA101) evokes homotypic adhesion and actin-dependent, lysosome-mediated cell death in B-cell malignancies. Blood 2011; 117:4519 - 29; http://dx.doi.org/10.1182/blood-2010-07-296913; PMID: 21378274
- Cragg MS, Glennie MJ. Antibody specificity controls in vivo effector mechanisms of anti-CD20 reagents. Blood 2004; 103:2738 - 43; http://dx.doi.org/10.1182/blood-2003-06-2031; PMID: 14551143
- Fransson J, Tornberg UC, Borrebaeck CA, Carlsson R, Frendéus B. Rapid induction of apoptosis in B-cell lymphoma by functionally isolated human antibodies. Int J Cancer 2006; 119:349 - 58; http://dx.doi.org/10.1002/ijc.21829; PMID: 16477633
- Zheng Y, Cai Z, Wang S, Zhang X, Qian J, Hong S, et al. Macrophages are an abundant component of myeloma microenvironment and protect myeloma cells from chemotherapy drug-induced apoptosis. Blood 2009; 114:3625 - 8; http://dx.doi.org/10.1182/blood-2009-05-220285; PMID: 19710503
- Zheng Y, Yang J, Qian J, Qiu P, Hanabuchi S, Lu Y, et al. PSGL-1/selectin and ICAM-1/CD18 interactions are involved in macrophage-induced drug resistance in myeloma. Leukemia 2013; 27:702 - 10; http://dx.doi.org/10.1038/leu.2012.272; PMID: 22996336