Abstract
Tumor recurrence may result from the exhaustion of tumor-associated antigen (TAA)-specific T cells, which often occurs upon chronic antigen stimulation. We have recently shown that the blockade of the T-cell inhibitory receptors PD-L1 and LAG-3 restores TAA-specific CD4+ T-cell functions in mice bearing recurrent neoplasms, resulting in robust antineoplastic effects.
Although tumor-associated antigen (TAA)-specific CD4+ T cells can eradicate established tumors in lymphopenic hosts,Citation1,Citation2 cancer may still recur. It is well known that recurrent tumors are more difficult to treat than primary lesions. The question is why. Recently, many mechanisms have been proposed in support of this notion, including the expansion of regulatory T cells (Tregs), the loss of antigen expression by malignant cells, the exhaustion of T cells, the development of adaptive tolerance, as well as the dedifferentiation of tumor cells.Citation3-Citation5
We have recently demonstrated that Treg-mediated immunosuppression and the chronic exhaustion of TAA-specific CD4+ T cells are deeply intertwined in the course of tumor recurrence.Citation6 Using a mouse model of melanoma combined with the adoptive transfer of CD4+ T cells and Tregs recognizing the melanoma differentiation antigen tyrosinase-related protein 1 (TYRP1),Citation2 we observed the accumulation of TAA-specific Tregs, the exhaustion of TAA-specific effector T cells, as well as an increase in the serum levels of the interferon γ (IFNγ)-inducible chemokines CXCL9 and CXCL10 during tumor recurrence. Programmed cell death 1 ligand 1 (PD-L1, also known as B7-H1 and CD274) blockade or Treg depletion could successfully treat primary neoplastic lesions in combination with adoptive cell transfer (ACT). However, neither supplemental therapy alone could reactivate TAA-specific CD4+ T cells during disease recurrence. In contrast, the blockade of PD-L1 combined with the depletion of TAA-specific Tregs resulted in the eradication of recurrent melanoma upon the successful transfer of TAA-specific CD4+ T cells. The combined blockade of the inhibitory molecules PD-L1 and lymphocyte-activation gene 3 (LAG-3) yielded similar results, which were confirmed by a decrease in the circulating concentration of CXCL9 and CXCL10.
We began by investigating the potential role of TAA-specific Tregs during cancer recurrence. Tregs expressing the diphtheria toxin receptor (DTR) under the control of the Foxp3 promoter were selectively ablated upon the injection of diphtheria toxin (DT). The removal of FOXP3+ Tregs early during primary tumor growth was not sufficient to prevent recurrence. Along similar lines, Treg depletion at the onset of tumor recurrence could not prevent the progression of relapsing lesions.
We next investigated the phenotypic and functional changes in TAA-specific CD4+ T cells during tumor recurrence. Relatively equal numbers of Tregs and effector T cells were observed in this setting, so we did not attribute tumor recurrence to a loss of effector T cells. Interestingly, both Tregs and effector CD4+ T cells from mice bearing recurring tumors expressed higher levels of programmed cell death 1 (PD-1), LAG-3, T-cell immunoreceptor with Ig and ITIM domains (TIGIT), and T-cell immunoglobulin mucin 3 (TIM-3) than the same cells isolated from mice with primary neoplasms. Additionally, we found decreased levels of the interleukin-7 receptor α (IL-7Rα) and CXCR3 on these cells. TAA-specific effector CD4+ T cells were functionally exhausted and produced only IFNγ, instead of both IFNγ plus tumor necrosis factor α (TNFα). Of note, we observed these effects regardless of the presence or absence of Tregs.Citation6 Our results indicated that TAA-specific CD4+ effector T cells were exhausted during tumor relapse ().
Figure 1. Combinatorial immunotherapeutic approaches for the treatment of recurrent tumors. (A) Interaction of the inhibitory receptors PD-1 and LAG-3 on CD4+ T cells with PD-L1 and MHC class II molecules, respectively, on antigen-presenting cells (APCs) and tumor cells leads to the de-phosphorylation of the T-cell receptor (TCR) and T-cell exhaustion. This corresponds to a robust decrease in T-cell proliferation, cytokine production and cytotoxic activity. (B) PD-L1-blocking antibodies reinvigorate T-cell activity (at least in part) by restoring TCR phosphorylation, while the blockade of LAG-3 either inhibits the immunosuppressive functions of regulatory T cells (Tregs) in a direct fashion or renders CD4+ effector T cells less susceptible to Tregs.
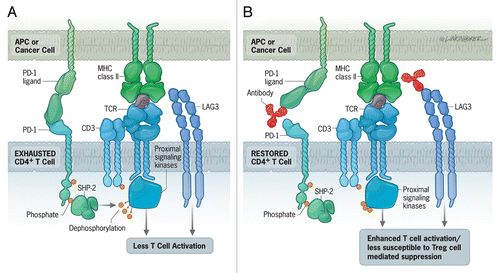
We sought to rescue exhausted CD4+ effector T cells by blocking inhibitory signals. We began by using a PD-L1-blocking antibody to interfere with the PD-1/PD-L1 signaling pathway. This treatment could not reverse CD4+ T-cell exhaustion, and recurrent tumors grew unabated in this setting. However, when we combined PD-L1 blockade with Treg depletion we obtained a dramatic reduction in the growth of recurring tumors.Citation6 Such a response developed, in some cases, within 2 weeks upon the administration of the combinatorial therapy. Following the restoration of anticancer immunity, the expression of inhibitory markers on TAA-specific CD4+ T cells decreased, while that of IL-7Rα and CXCR3 increased. In this setting, TAA-specific CD4+ T cells began to produce TNFα along with IFNγ.
The reduced expression of inhibitory receptors on effector CD4+ T cells following the administration of anti-PD-L1 antibodies coupled to Treg depletion implies a reduction in inhibitory signaling. We therefore reasoned that the direct inhibition of such inhibitory signal transduction cascades might also efficiently treat relapsing tumors. To test this hypothesis, we combined a LAG-3-blocking antibody with the anti-PD-L1 antibody (). The effects of the combined blockade of PD-L1 and LAG-3 were comparable to those of Treg depletion plus PD-L1 blockade.Citation6 As anti-PD-L1 and anti-LAG-3 antibodies are already being tested in Phase I clinical trials, these findings are rather easily translatable to the clinical management of patients with recurrent tumors. Surprisingly, the combination of LAG-3 blockade with Treg depletion did not reinvigorate CD4+ T cells.Citation6 It appears therefore that the blockade of LAG-3 specifically affects Tregs and must be combined with anti-PD-L1 strategies for achieving a therapeutic effect against relapsing tumors. This demonstrates that tolerance is enforced by multiple, non-redundant mechanisms in vivo. Moreover, PD-L1-targeting approaches alone may actually activate both PD-1+ effector T cells and Tregs.
IFNγ-producing cells are known to dampen immune responses, at least in some circumstances, by causing adaptive resistance.Citation7 We postulated that the increased circulating levels of CXCL9 and CXCL10 might be a harbinger of adaptive resistance. Increased concentrations of CXCL9 and CXCL10 in the serum may indeed initiate a negative feedback loop, resulting in the downregulation of CXCR3 expression on TAA-specific CD4+ T cells. Moreover, high levels of these chemokines may potentially “drown” CD4+ T cells and hence prevent them from accessing the tumor microenvironment. Thus in line with the aphorism that hung over the Oracle at Delphi in ancient Greece, “Nothing in excess.” After successful combination therapy, the serum levels of these chemokines returned to homeostatic concentrations.
We propose that during the growth of primary tumors, when the amount of Tregs and the expression levels of inhibitory receptors on effector T cells are relatively low, ACT combined with a supplemental therapy can mediate effective, long-term protection. Upon recurrence, however, the immune system becomes overwhelmed by several inhibitory cues. Thus, in this setting, treatment must rely on interventions that attempt to overcome multiple layers of tolerance.
Combination therapies based on anti-PD-L1 and anti-LAG-3 antibodies have shown promise in mouse models of chronic infectious diseases, such as lymphocytic choriomeningitis (LCM)Citation8 and malaria.Citation9 To our knowledge, we were the first to demonstrate that the combinatorial blockade of PD-L1 and LAG-3 represents a promising strategy for the treatment of recurrent tumors.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Quezada SA, Simpson TR, Peggs KS, Merghoub T, Vider J, Fan X, et al. Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med 2010; 207:637 - 50; http://dx.doi.org/10.1084/jem.20091918; PMID: 20156971
- Xie Y, Akpinarli A, Maris C, Hipkiss EL, Lane M, Kwon EK, et al. Naive tumor-specific CD4(+) T cells differentiated in vivo eradicate established melanoma. J Exp Med 2010; 207:651 - 67; http://dx.doi.org/10.1084/jem.20091921; PMID: 20156973
- Landsberg J, Kohlmeyer J, Renn M, Bald T, Rogava M, Cron M, et al. Melanomas resist T-cell therapy through inflammation-induced reversible dedifferentiation. Nature 2012; 490:412 - 6; http://dx.doi.org/10.1038/nature11538; PMID: 23051752
- Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol 2012; 24:207 - 12; http://dx.doi.org/10.1016/j.coi.2011.12.009; PMID: 22236695
- Jensen SM, Twitty CG, Maston LD, Antony PA, Lim M, Hu HM, et al. Increased frequency of suppressive regulatory T cells and T cell-mediated antigen loss results in murine melanoma recurrence. J Immunol 2012; 189:767 - 76; http://dx.doi.org/10.4049/jimmunol.1103822; PMID: 22723522
- Goding SR, Wilson KA, Xie Y, Harris KM, Baxi A, Akpinarli A, et al. Restoring Immune Function of Tumor-Specific CD4+ T Cells during Recurrence of Melanoma. J Immunol 2013; 190:4899 - 909; http://dx.doi.org/10.4049/jimmunol.1300271; PMID: 23536636
- Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 2012; 4:27ra37; http://dx.doi.org/10.1126/scitranslmed.3003689; PMID: 22461641
- Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol 2009; 10:29 - 37; http://dx.doi.org/10.1038/ni.1679; PMID: 19043418
- Butler NS, Moebius J, Pewe LL, Traore B, Doumbo OK, Tygrett LT, et al. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nat Immunol 2012; 13:188 - 95; http://dx.doi.org/10.1038/ni.2180; PMID: 22157630