Abstract
A large unmet need exists for cost-effective, widely available antineoplastic immunotherapeutic agents with a robust translational potential. MicroRNAs (miRNAs) that regulate tumor-mediated immunosuppression or immune checkpoints can induce robust therapeutic immune responses, indicating that miRNAs may ultimately become part of the portfolio of anticancer immunotherapeutics.
MicroRNAs (miRNAs) are short, non-coding RNAs that modulate protein expression, are altered in the course of oncogenesis and play a key role in the proliferation, invasiveness, metastatic potential and resistance to therapy of cancer cells. One of the major limitations of using miRNAs as therapeutic approaches against cancer is to obtain an adequate delivery to neoplastic lesions. This is especially germane for tumors of the central nervous system (CNS), which are protected by the blood-brain barrier. Conversely, there is less concern in this respect about antitumor immunotherapeutic approaches, as these can diffusely access tumors, including those located within the CNS. The immune system can indeed recognize and eradicate tumors, but this is highly mitigated by a plethora of tumor-mediated immunosuppressive mechanisms. Over the last decade, immunologists have redirected their efforts toward blocking tumor-mediated immunosuppression as a means to generate therapeutically relevant antitumor immune responses. Thus, we reasoned that if miRNAs could be used to reverse tumor-mediated immunosuppression and hence induce antitumor immunity so that the delivery issue would be overcome, the immune system would become a “Trojan horse” and mediate miRNA-ignited therapeutic effects. This is particularly relevant as circulating immune cells are among the first cell population to be exposed to intravenously administered miRNAs.
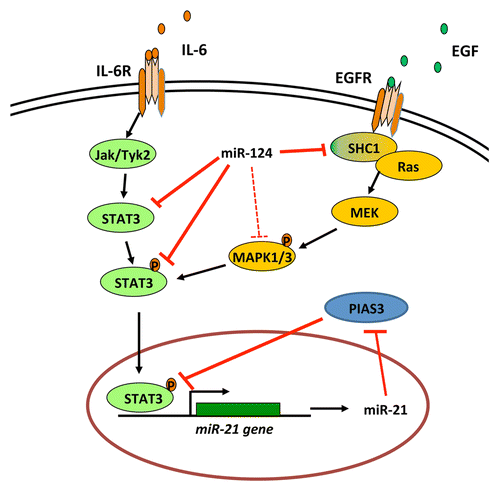
To identify potential immunomodulatory miRNAs, we utilized a step-wise process of (1) screening for miRNAs that are downregulated in malignant or tumor-associated immune cells relative to their normal counterparts; (2) evaluating such candidate miRNAs for their potential effects on immunosuppressive pathways; and (3) then validating the miRNA targets and testing the therapeutic effects of miRNAs in immunocompetent model systems. Because the signal transducer and activator of transcription 3 (STAT3) has been shown to play a key role in a wide variety of tumor-mediated mechanisms of immunosuppression,Citation1-Citation3 STAT3 was one of the first candidates that we assessed for potential miRNA binding. We found that miR-124 targets multiple nodes of the STAT3 signaling pathway (). In line with this notion, both the systemic administration of miR-124 or the adoptive transfer of miR-124-transfected T cells exerted potent therapeutic effects in clonotypic and genetically engineered murine models of glioblastoma and robustly enhanced immune effector responses within the tumor microenvironment. These effects were ablated in both CD4+ and CD8+ cell-depleted mice as well as in nude mice, indicating that miR-124 exerts antineoplastic activities that depends on a T cell-mediated immune response.Citation4
Figure 1. Inhibitory activity of miR-124 on multiple nodes of the STAT3 signaling pathway. Signal transducer and activator of transcription 3 (STAT3) can be activated by a variety of ligands, including interleukin-6 (IL-6) and the epidermal growth factor (EGF). miR-124 not only can downregulate signal transducers like SHC1 and STAT3, but also can occasionally inhibit the phosphorylation of mitogen-activated protein kinases 1 and 3 (MAPK1 and MAPK3), probably in a contextual fashion, when IL-6 is absent and cells are highly dependent on EGF receptor (EGFR) signaling.
The concept of using miRNAs to modulate immune responses is not only centered around STAT3. We have now identified miRNAs that target transforming growth factor β receptor 1 (TGFBR1), such as miR-142–3p, which disrupts autocrine stimulation and results in the selective apoptotic demise of tumor-supportive M2 macrophages. Accordingly, miR-142–3p drives effective anticancer immune responses in established, heterogeneous, genetically engineered, high-grade glioma models.Citation5 Our strategy to identify immunomodulatory miRNAs is now being used to identify additional therapeutic molecules that may target other immunosuppressive effectors including (but not limited to) interleukin-10 (IL-10), programmed cell death 1 (PDCD1, best known as PD-1), PDCD1 ligand 1 (PDCD1L1, best known as PD-L1 or CD174), cytotoxic T-lymphocyte antigen 4 (CTLA4) and forkhead box P3 (FOXP3). There are far-reaching implications for our identification strategy, as we have demonstrated that miRNAs can reverse tumor-elicited immunosuppressive mechanisms in a therapeutically relevant fashion. Reciprocally, by screening the immune cells of patients bearing autoimmune diseases relative to those of normal subjects, miRNAs could be identified that may block pro-inflammatory responses, perhaps leading to the development of immunomodulatory agents for the treatment of multiple sclerosis, rheumatoid arthritis, psoriasis or graft rejection.
There are several unique advantages in using miRNAs for immunomodulation. Unlike other proprietary agents including monoclonal antibodies or chemotherapeutics, miRNAs are indeed readily available to the scientific community. Furthermore, miRNAs allow for targeting intracellular molecule, whereas antibody-based immunotherapies are limited to extracellular or cell-surface exposed targets. Moreover, although a variety of cell-based immunotherapeutic approaches are undergoing clinical testing for their antineoplastic potential, with the evolution of cost-effective medicine, inexpensive immunomodulatory agents hold great commercial appeal. In this respect, miRNAs are easy and inexpensive to manufacture. In addition, alternative approaches such as the use small molecule inhibitors generally induce modest or transient responses as a consequence of redundant signal transduction pathways or the activation of compensative signals.Citation6,Citation7 miRNAs are known to target networks, and we found that single miRNAs can block multiple nodes of a given signaling pathway including those activated by alternative ligands (). This may provide a unique therapeutic advantage. Finally, although there are small inhibitors of the STAT3 signaling pathway that will soon be entering clinical trials,Citation8 the toxicity profile of miR-124 may be favorable as compared with that of chemotherapeutic agents, as miR-124 is an endogenous biological product that is normally expressed by most cell types, including neurons.
Limitations to the use of miRNAs as therapeutic agents, reflecting their biological complexity, are the elucidation of relevant biomarkers for clinical trials and the establishment of a comprehensive understanding of their mechanism of action. The mechanistic targets of miRNAs are likely to be context- and cell-specific and their precise elucidation may require extensive pre-clinical studies. For example, in the course of autoimmune responses miR-124 probably targets C/EBP-α-PU.1 and hence deactivates immune effectors,Citation9 whereas in the context of antitumor immunity it inhibits immunosuppressive STAT3 and thus activates the immune system.
In the future, as the immunosuppressive mechanisms employed by cancer to evade immunosurveillance are heterogeneous,Citation10 specific tumors may be screened for operational immunosuppressive pathways, leading to the selection of the appropriate miRNA(s) for use. Alternatively, tumor-mediated immunosuppression could be comprehensively controlled in unselected patients by using a combination of multiple miRNAs. Ultimately, miRNAs may serve as a new class of immunotherapeutics with distinct advantages, but achieving this goal requires a substantial investment and effort from multiple investigators.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Kortylewski M, Kujawski M, Wang T, Wei S, Zhang S, Pilon-Thomas S, et al. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat Med 2005; 11:1314 - 21; http://dx.doi.org/10.1038/nm1325; PMID: 16288283
- Wei J, Barr J, Kong LY, Wang Y, Wu A, Sharma AK, et al. Glioblastoma cancer-initiating cells inhibit T-cell proliferation and effector responses by the signal transducers and activators of transcription 3 pathway. Mol Cancer Ther 2010; 9:67 - 78; http://dx.doi.org/10.1158/1535-7163.MCT-09-0734; PMID: 20053772
- Wu A, Wei J, Kong LY, Wang Y, Priebe W, Qiao W, et al. Glioma cancer stem cells induce immunosuppressive macrophages/microglia. Neuro Oncol 2010; 12:1113 - 25; http://dx.doi.org/10.1093/neuonc/noq082; PMID: 20667896
- Wei J, Wang F, Kong LY, Xu S, Doucette T, Ferguson SD, et al. miR-124 inhibits STAT3 signaling to enhance T cell-mediated immune clearance of glioma. Cancer Res 2013; In press http://dx.doi.org/10.1158/0008-5472.CAN-12-4318; PMID: 23636127
- Xu S, Wei J, Wang F, Kong L-Y, Ling X-Y, Doucette TA, et al. miR-142-3p inhibits the M2 macrophage and exerts therapeutic efficacy against murine glioblastoma. Journal of National Cancer Institute
- Fan QW, Knight ZA, Goldenberg DD, Yu W, Mostov KE, Stokoe D, et al. A dual PI3 kinase/mTOR inhibitor reveals emergent efficacy in glioma. Cancer Cell 2006; 9:341 - 9; http://dx.doi.org/10.1016/j.ccr.2006.03.029; PMID: 16697955
- Mellinghoff IKWM, Wang MY, Vivanco I, Haas-Kogan DA, Zhu S, Dia EQ, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med 2005; 353:2012 - 24; http://dx.doi.org/10.1056/NEJMoa051918; PMID: 16282176
- Hussain SF, Kong LY, Jordan J, Conrad C, Madden T, Fokt I, et al. A novel small molecule inhibitor of signal transducers and activators of transcription 3 reverses immune tolerance in malignant glioma patients. Cancer Res 2007; 67:9630 - 6; http://dx.doi.org/10.1158/0008-5472.CAN-07-1243; PMID: 17942891
- Ponomarev ED, Veremeyko T, Barteneva N, Krichevsky AM, Weiner HL. MicroRNA-124 promotes microglia quiescence and suppresses EAE by deactivating macrophages via the C/EBP-α-PU.1 pathway. Nat Med 2011; 17:64 - 70; http://dx.doi.org/10.1038/nm.2266; PMID: 21131957
- Doucette TA, Rao G, Rao A, Shen L, Aldape K, Wei J, et al. Immune heterogeneity of glioblastoma subtypes: extrapolation from the cancer genome atlas. Cancer Immunology Research